Heavy metals are chemical elements with a density of more than 5 g/cm³ and a high atomic weight. Due to their specific physical and chemical properties, they are used in numerous industrial processes. The most common heavy metals in industrial wastewater include lead (Pb), copper (Cu), zinc (Zn), nickel (Ni), chromium (Cr), cadmium (Cd) and mercury (Hg). However, these metals are toxic even in low concentrations and therefore pose a considerable challenge for water and wastewater treatment.
Table of contents
Industrial sources of heavy metals
The industrial use of heavy metals leads to their entry into wastewater. This pollution depends heavily on the respective processes and industry. Heavy metals can enter the environment through wastewater, rinsing water or production residues. The most important industrial sources are described below:
Electroplating and surface treatment
- Application:
- Nickel, chromium and zinc are used in electroplating to coat metals in order to achieve corrosion protection, hardness or decorative properties.
- Heavy metals in wastewater:
- Typical contaminants are chromium(VI), nickel, zinc and copper.
- Challenges:
- Rinsing water often contains a combination of different heavy metals, which makes targeted separation difficult.
Metal processing
- Steel and aluminum production:
- Residues from metal smelting and metal processing contain heavy metals such as cadmium, zinc and lead.
- Toolmaking and mechanical engineering:
- Wastewater often contains copper and nickel contamination from lubricants and coolants as well as from production technology.
- Automotive industry:
- Heavy metals such as zinc, chromium and copper are released into wastewater during vehicle production, particularly during painting, coating and the manufacture of components.
Mining and ore processing
- Application:
- The mining of metals such as copper, zinc or lead and the processing of ores produce wastewater with high concentrations of iron, manganese, cadmium and other metals.
- Challenges:
- Wastewater from mines is often acidic (so-called "acid mine drainage") and contains heavy metals in dissolved form.
Chemical and petrochemical industry
- Application:
- Heavy metals such as mercury, nickel and chromium are used as catalysts in chemical reactions.
- Heavy metals in wastewater:
- Residues from catalytic processes and impurities in raw materials contribute to heavy metal pollution.
Electronics and electrical industry
- Application:
- Heavy metals such as copper, lead and tin are essential for the production of printed circuit boards, batteries and electronic components.
- Heavy metals in wastewater:
- Soldering materials, galvanic processes and the cleaning of components lead to exposure to lead, zinc, nickel and copper.
Paint, varnish and pigment industry
- Application:
- Heavy metals such as chromium, lead and cadmium are used as colour pigments and stabilizing agents.
- Heavy metals in wastewater:
- Wastewater often contains residues from production that are toxic and difficult to biodegrade.
Glass and ceramics industry
- Application:
- Heavy metals such as lead and cadmium are used in the manufacture of glass and ceramic glazes.
- Challenges:
- The wastewater often contains metal compounds that are difficult to dissolve and require special treatment.
Effects of heavy metals on the environment and health
Toxicity and bioaccumulation
Heavy metals are not biodegradable and accumulate in the environment. Even small quantities can cause considerable damage to ecosystems:
- Chromium(VI): Highly toxic and carcinogenic; frequently used in galvanic processes.
- Nickel: Irritant and allergenic; a common contaminant in industrial wastewater.
- Mercury: Neurotoxic and extremely persistent; bioaccumulates in aquatic organisms.
- Cadmium: Causes kidney and bone damage; often found in batteries and pigments.
Effects on technical systems
- Corrosion:
- Heavy metals in water promote electrochemical corrosion in pipes and systems.
- Disruption of biological processes:
- Heavy metals inhibit microorganisms that break down organic substances in biological sewage treatment plants and thus disrupt the purification performance.
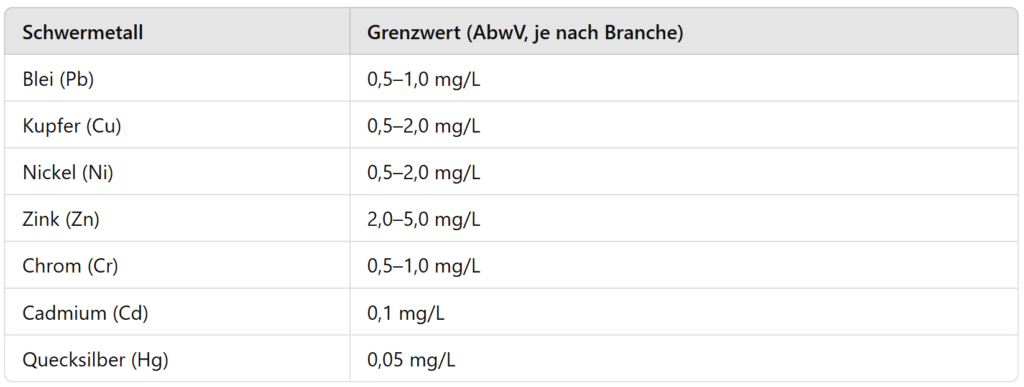
Limit values for heavy metals in industrial wastewater
The discharge of heavy metals into bodies of water or public wastewater systems is strictly regulated. The Wastewater Ordinance (AbwV) sets industry-specific limits that must be adhered to in order to minimize environmental pollution.
Process for removing heavy metals from water and wastewater
The choice of treatment technology depends on the concentration, the type of heavy metal and the water quality requirements. CP systems are often combined with ion exchangers or activated carbon filters.
1. precipitation and flocculation in CP plants
Chemical precipitation is a key process in water and wastewater treatment for the removal of heavy metals. The process is based on the chemical conversion of dissolved heavy metal ions into poorly soluble compounds, which can then be removed from the water by sedimentation, flotation or filtration.
Mechanism of precipitation
Reaction with hydroxide ions (hydroxide precipitation):
- The addition of alkalis such as milk of lime (Ca(OH)₂) or caustic soda (NaOH) leads to an increase in the pH value, causing heavy metal ions to precipitate in the form of sparingly soluble metal hydroxides.
- This produces metal hydroxide precipitates, which can be separated from the water due to their low solubility.
Reaction with sulphides (sulphide precipitation):
- For heavy metals with particularly low solubility as sulphide (e.g. mercury, lead), sulphide ions are used, typically from sodium sulphide (Na₂S).
- Sulphides have the advantage that they form stable precipitates even at slightly acidic pH values.
Precipitation with complexing agents:
- In cases where heavy metals are present in stable complexes (e.g. due to chelating agents such as EDTA), special reagents may be required to break down the complexes before precipitation takes place.
Factors influencing the precipitation
The effectiveness of precipitation depends on various chemical and physical factors that must be carefully controlled:
pH value:
- The pH value is the most important parameter, as the solubility of most heavy metal hydroxides is strongly dependent on the pH value.
- Examples:
- Zinc hydroxide precipitates optimally at pH 9-10.
- Iron(III) hydroxide precipitates at a pH value of 6-8.
- Precise control of the pH value is necessary, as other metals (e.g. aluminum) can become soluble again if the pH value is too high.
Concentration of the reagents:
- A sufficient amount of precipitant is required to bind all heavy metal ions. However, overdosing can lead to increased operating costs and additional chemical consumption.
Temperature:
- The reaction rate and the solubility of the precipitation products are temperature-dependent. Higher temperatures generally promote the reaction rate.
Stirring intensity and contact time:
- Good mixing ensures intensive contact between the reagents and the heavy metal ions.
- The contact time must be long enough for the chemical reactions to take place completely.
Interference from other ions:
- Anions such as chloride or sulphate can increase the solubility of certain heavy metal compounds and thus reduce the efficiency of precipitation.
- In such cases, additional precipitation reagents or pre-treatment steps are required.
Flocculation: stabilization and separation of precipitation products
After precipitation, the precipitation products produced often remain dispersed in the water as fine particles. Flocculation is used to combine these particles into larger aggregates (flocs), which are easier to settle or separate.
Mechanism of flocculation
- Coagulation:
- By adding coagulants such as iron(III) or aluminum salts, the electrostatic repulsive forces between the particles are reduced, which facilitates their agglomeration.
- Flocculation:
- Flocculants such as polymers (e.g. polyacrylamides) increase particle formation by binding the flocs together through bridging.
Limits of precipitation and flocculation
Complexed heavy metals:
- Heavy metals that are present in stable organic or inorganic complexes are difficult to precipitate. Prior splitting of the complexes (e.g. by oxidation or reduction) is necessary.
Residual concentrations:
- Die chemische Fällung erreicht häufig nicht die extrem niedrigen Restkonzentrationen (< 0,01 mg/l) , die in manchen Industrien erforderlich sind. In solchen Fällen sind ergänzende Verfahren wie Adsorption oder Ionenaustausch erforderlich.
Sludge production:
- Precipitation generates large quantities of precipitation sludge, which must be treated and disposed of as hazardous waste. The disposal costs can be considerable.
Chromium(VI):
- Heavy metals such as chromium(VI) must be reduced to the less toxic form chromium(III) before precipitation, for example by adding sodium bisulphite.

Photo: Our CP system ALMA CHEM MCW with precipitation and flocculation, sludge dewatering and downstream multi-layer filter and ion exchanger (if required)
2. ion exchange
Ion exchange is a physico-chemical process in which dissolved ions in water are replaced by ions in a solid ion exchange resin. The process is based on the specific binding of cations or anions to active groups in a resin material. Due to the high selectivity of the resins, heavy metals can be removed even from highly diluted solutions.
Mechanism of ion exchange
- Cation exchange:
- Heavy metal cations such as Cu²⁺, Zn²⁺ or Pb²⁺ are replaced by H⁺ ions (hydrogen-based resins) or Na⁺ ions (sodium-based resins).
- The heavy metal ion is bound to the resin matrix and removed from the water.
- Anion exchange:
- Negatively charged heavy metal complexes, such as chromates (CrO₄²-), are replaced by OH- ions.
Types of ion exchangers
Strong cation exchangers:
- Effective in removing heavy metals such as copper, nickel and zinc from acidic solutions.
- Can be used at pH values from 1 to 14.
Weak cation exchangers:
- Effective at medium to high pH values; ideal for slightly acidic to neutral solutions.
Strong anion exchangers:
- Remove negatively charged complexes such as chromates or arsenates.
- Use in alkaline solutions.
Selective resins:
- Developed for specific heavy metals such as mercury, cadmium or chromium.
Factors influencing the ion exchange
ion concentration:
- Ion exchange is particularly efficient at low concentrations, as the resins have a high affinity for heavy metal ions.
pH value:
- Der pH-Wert beeinflusst die Ladung der Schwermetalle und somit ihre Bindung an das Harz. Beispielsweise liegt Eisen bei pH < 3 als Fe³⁺ vor und kann leicht entfernt werden, während es bei höheren pH-Werten zu Hydroxid fällt.
Competition:
- The presence of other cations such as Ca²⁺ or Mg²⁺ can reduce the effectiveness of the ion exchange.
Resin capacity:
- The maximum load of the resin is limited by its specific capacity (equivalent ions per volume).
Regeneration:
- Once the capacity limit has been reached, the resin is regenerated using chemicals such as hydrochloric acid (HCl) or caustic soda (NaOH).
Advantages and limitations of ion exchange
Advantages:
- Very high selectivity, even for low concentrations of heavy metals.
- Regenerable, which reduces operating costs.
- Can be customized for specific metals (e.g. chrome or copper).
Limits:
- Limited capacity at high concentrations.
- Competition from other ions can reduce efficiency.
- Regeneration chemicals generate additional waste water.

Photo: Our ALMA ION ion exchanger system with upstream ALMA FIL AK activated carbon filter
3. activated carbon adsorption
Adsorption using activated carbon filters is based on the physical or chemical adsorption of heavy metal ions or heavy metal complexes on the surface of a porous material such as activated carbon. The large inner surface of the activated carbon is used to bind the dissolved substances from the water.
Mechanism of adsorption
- Physical adsorption:
- Heavy metals are held on the surface of the activated carbon by van der Waals forces or electrostatic interactions.
- Chemisorption:
- Heavy metals form chemical bonds with functional groups on the surface of the activated carbon (e.g. carboxyl or hydroxyl groups).
Types of activated carbon
Powdered activated carbon (PAH):
- Fine powder that is added directly to the water.
- Particularly suitable for batch treatments.
Granular activated carbon (GAK):
- Coarse-grained activated carbon used in filters.
- Long-term and continuous use possible.
Impregnated activated carbon:
- Treated with chemical reagents (e.g. sulphur) to efficiently remove specific heavy metals such as mercury or arsenic.
Factors influencing adsorption
concentration of heavy metals:
- Higher concentrations lead to better utilization of the adsorption capacity, but also to faster saturation.
pH value:
- The pH value influences the charge of the heavy metal ions and the surface charge of the activated carbon.
- Example:
- At a low pH value, heavy metals such as Pb²⁺ are positively charged and bind well to the negatively charged activated carbon.
Temperature:
- Adsorption processes are usually exothermic, so increasing the temperature can reduce efficiency.
Pore structure:
- The size and distribution of the pores in the activated carbon influence the binding capacity.
Optimization of adsorption
- Pre-treatment of the water:
- Removal of suspended solids and organic compounds improves effectiveness.
- Use of impregnated activated carbon:
- Specially treated activated carbon is much more effective for heavy metals such as arsenic and mercury.
- Regeneration:
- The saturation of the activated carbon can be reversed by thermal or chemical processes, which reduces operating costs.
Advantages and limitations of activated carbon adsorption
Advantages:
- High efficiency with low heavy metal concentrations.
- Versatile, can also be used for other pollutants such as organic compounds.
- Can be impregnated for specific heavy metals.
Limits:
- Limited capacity of the adsorption materials.
- Regeneration is energy and chemical intensive.
- Less suitable for high concentrations of heavy metals.

Photo: Our ALMA FIL AK activated carbon filters with upstream ALMA FIL multi-layer filter
Conclusion
The treatment of wastewater contaminated with heavy metals from industrial processes is one of the central challenges of water treatment. The specific requirements for wastewater treatment vary greatly depending on the industry and production process. With advanced technologies such as precipitation and flocculation in CP plants, ion exchange and activated carbon adsorption, the legal limits can be reliably met and the environmental impact minimized.
For further information on our products, please feel free to contact us at any time!