PFAS (per- and polyfluoroalkyl substances) are a group of synthetic organic compounds that are used in numerous industrial and commercial applications due to their unique chemical and physical properties. At the same time, their persistence, mobility and toxicological effects make them a major challenge for industrial water and wastewater treatment.
PFAS are also known as "forever chemicals" because they are hardly degraded in the environment and can accumulate in organisms as well as in water and soil resources. Their removal requires specialized processes and technologies.
Table of contents
Chemical and physical properties of PFAS
PFAS are characterized by the following properties:
Chemical structure:
- Consist of a chain of carbon atoms that are fully or partially substituted with fluorine atoms.
- The C-F bond is one of the strongest bonds in chemistry and gives PFAS its thermal stability, chemical resistance and hydrophobicity.
Persistence:
- PFAS are extremely stable against biological and chemical degradation.
- Their half-life in the environment and in living organisms is several years to decades.
Hydrophilic and lipophilic properties:
- PFAS are amphiphilic, i.e. they have both water-repellent (hydrophobic) and grease-repellent (lipophobic) properties.
Mobility:
- Long chains such as PFOA (perfluorooctanoic acid) or PFOS (perfluorooctane sulfonate) are highly mobile and can contaminate groundwater and surface water over long distances.
Sources of PFAS
PFAS are used in a variety of products and processes, including
1. industrial processes
PFAS are used in industrial processes to utilize specific functional properties such as chemical stability, hydrophobicity and temperature resistance. Here are some of the main applications:
a) Production of fire-fighting foams (AFFF)
- PFAS-based Aqueous Film Forming Foams (AFFF) are used to extinguish rapidly flammable liquids such as gasoline or oil.
- They form a chemically stable foam layer that separates oxygen from the source of the fire and makes firefighting effective.
- Areas of application: Airports, petrochemical plants, military facilities.
- Problem: Residues of these foams often get directly into the soil or groundwater and spread from there over large areas.
b) Electroplating and surface treatment
- PFAS are used as wetting agents in electroplating to minimize spattering during the coating process and to produce even layers on metal surfaces.
- Typical applications: Production of anti-corrosion coatings and durable metal products.
- Danger: Production wastewater often contains high concentrations of PFAS, which can enter the environment without treatment.
c) Textile and leather industry
- PFAS are used as water, dirt and grease-repellent coatings in textiles, leather goods and carpets.
- Examples: Impregnated outdoor clothing, water-repellent shoes, stain-resistant sofa covers.
- Environmental problem: Waste and residues from production and the leaching of PFAS during use and cleaning.
2. consumer products
Many everyday products contain PFAS because they make them more durable, resistant and functional. Some main applications are:
a) Non-stick coatings in cookware
- PFAS, in particular PTFE (polytetrafluoroethylene), is used for non-stick coatings in pots and pans.
- Advantages: Increased heat resistance and easy cleaning.
- Problem: Wear of the coating during use can release small amounts of PFAS, which can end up in the environment or in food.
b) Impregnations for paper, cardboard and packaging
- PFAS give paper products water and grease repellent properties.
- Examples: Packaging for fast food products, pizza boxes, microwave popcorn bags.
- Danger: After use, this packaging ends up in the waste and releases PFAS during decomposition, e.g. in landfill leachate.
c) Cleaning agents and polishes
- PFAS-containing products are used to produce water and dirt-repellent coatings for surfaces such as glass, metal and ceramics.
- Example: vehicle polishes, window cleaners, protective agents for furniture.
- Problem: PFAS are released into the wastewater through use and subsequent rinsing.
3. wastewater and landfills
PFAS from industrial processes and consumer products are ultimately released into the environment:
a) Production wastewater
- Wastewater from industrial plants often contains high concentrations of PFAS, especially if it is discharged into water bodies without adequate treatment.
- Industries with particularly high emissions: chemical production, textile factories, metal processing and plastics production.
b) Landfill leachate
- PFAS-contaminated materials such as impregnated packaging, textiles or residues from fire extinguishing foam are often stored in landfills.
- When this waste is broken down, PFAS are released into the leachate, which can enter the groundwater or surface water if left untreated.
- Challenge: The mobility of PFAS makes recovery from leachate difficult.
c) Diffuse sources
- PFAS are also released from diffuse sources, for example through atmospheric input or agricultural areas that have been fertilized with PFAS-contaminated sewage sludge.
- These inputs are difficult to control and contribute to large-scale contamination.
Challenges posed by PFAS in water and wastewater treatment
Health effects:
- PFAS are bioaccumulative and are suspected of being carcinogenic, endocrine disrupting and immunotoxic.
- They influence reproduction, the hormone system and the immune system.
Regulatory requirements:
- Strict legal limits for PFAS in drinking water and wastewater are already in force or are being introduced worldwide.
- In the EU, for example, a maximum of 0.1 µg/L applies to PFOA and PFOS in drinking water.
Technological challenges:
- PFAS are difficult to degrade and cannot be removed by conventional water or wastewater treatment processes such as biological clarification or simple filtration.
Technologies for the removal of PFAS
The removal of PFAS from water requires specialized and innovative processes. The most important technologies include
1. adsorption by activated carbon
- Mechanism: PFAS molecules are adsorbed on the porous surface of the activated carbon.
- Advantages: High efficiency in the removal of long-chain PFAS (e.g. PFOS, PFOA).
- Limitations: Less effective with short-chain PFAS, which are less hydrophobic. Regular regeneration or replacement of the activated carbon required.

Photo: Our ALMA FIL AK activated carbon filters
2. ion exchanger systems
- Mechanism: PFAS are bound to resin surfaces through ionic interactions.
- Advantages: Very effective for short-chain PFAS.
- Restrictions: High operating costs and regular regeneration.
3. oxidation process
- UV/ozone, Fenton or UV/H2O2:
- Partial decomposition of PFAS by highly reactive hydroxyl radicals.
- Effective for certain PFAS such as PFOS, but not for all.
- Restrictions:
- High energy requirements.
- Risk of formation of toxic intermediates
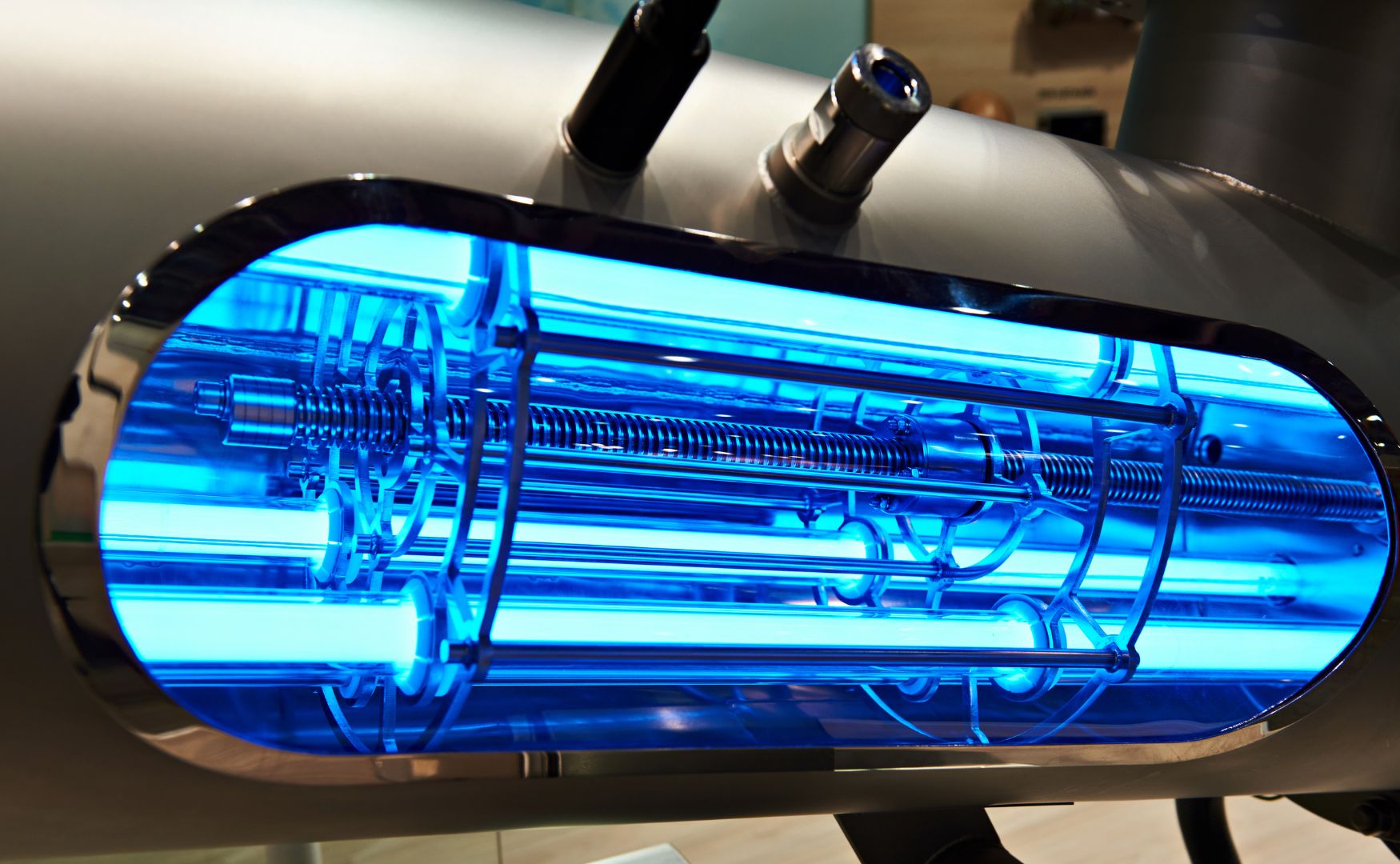
Photo: UV reactor in conjunction with the dosing of ozone or hydrogen peroxide in our ALMA OXI UV system
4. membrane process
- Reverse osmosis (RO) and Nanofiltration (NF):
- Mechanical barrier that retains PFAS molecules.
- Effective removal of almost all PFAS, regardless of chain length.
- Restrictions:
- Generation of a PFAS-containing concentrate stream that requires further treatment.
- High energy and maintenance costs.

Photo: Our ALMA OSMO Process reverse osmosis system for removing PFAS, installed in the ALMA MODUL technical room container
5. chemical-physical treatment
CP plants combine several physico-chemical processes to effectively remove PFAS and other trace substances from wastewater. Processes such as precipitation, flocculation, neutralization, filtration and oxidation are used. The efficiency of this method is particularly evident in the pre-treatment of industrial wastewater with high concentrations of pollutants.
Advantages:
- High flexibility in the removal of organic and inorganic trace substances.
- Effective pre-treatment for downstream processes such as membrane technology or biological systems.

Process integration and operational management
In industrial plants, a combination of several technologies is often used to ensure effective removal of PFAS:
Pre-treatment:
- Mechanical filtration to remove solids.
- Activated carbon or ion exchanger for pre-cleaning.
Main treatment:
- Reverse osmosis to remove PFAS from the main stream.
- Oxidation processes (AOP process) for further decomposition.
Follow-up treatment:
- Treatment of concentrate streams (e.g. thermal decomposition or further adsorption).
- Return of purified water to the process cycle.
Monitoring and process control:
- Regular monitoring using HPLC-MS/MS (High Performance Liquid Chromatography coupled with Mass Spectrometry) to determine PFAS concentrations.
- Use of online analyzers for continuous process monitoring.
Economic and ecological aspects
Operating costs:
- Technologies such as activated carbon and reverse osmosis are energy-intensive, especially for the regeneration or treatment of residues.
Disposal:
- Residues, such as loaded activated carbon or concentrates, must be properly disposed of or further treated.
Conclusion
PFAS pose a considerable challenge for industrial water and wastewater treatment. Due to their persistence and mobility, they require specialized processes such as adsorption, membrane filtration or oxidation. The integration of such technologies into existing plants and compliance with strict legal requirements are essential in order to minimize the environmental impact of PFAS and ensure sustainable water quality. In view of the increasing requirements, the development of innovative technologies will be of central importance in the coming years.
For further information on our products, please feel free to contact us at any time!








