Heavy metal reduction refers to the process of removing or minimizing heavy metals in water and wastewater systems. Heavy metals such as copper (Cu), nickel (Ni), chromium (Cr), zinc (Zn), cadmium (Cd) and lead (Pb) are one of the biggest challenges in water and wastewater treatment due to their toxicity and persistence in the environment. Their efficient reduction is crucial in order to comply with legal limits, protect the environment and ensure water quality for industrial processes.
Table of contents
Technical background and characteristics of heavy metals
Chemical properties of heavy metals
- Solubility:
- Heavy metals can be present as dissolved ions, complexes or particles. Their solubility depends strongly on the pH value and the chemical environment.
- Example: Chromium(VI) is highly soluble in neutral to alkaline water, while chromium(III) precipitates as a hydroxide.
- Oxidation states:
- Some heavy metals such as chromium or iron occur in different oxidation states, which influence the choice of reduction process.
- Complex formation:
- Heavy metals can form stable complexes with organic substances (e.g. EDTA), which makes them difficult to remove.
Challenges in heavy metal reduction
- High toxicity:
- Heavy metals have a toxic effect on aquatic ecosystems and are harmful to human health.
- Regulatory requirements:
- Compliance with legal limits is often only possible with complex and multi-stage processes.
- Diversity of wastewater composition:
- Industrial wastewater can contain high concentrations of heavy metals, organic substances and other contaminants that require specific treatment approaches.
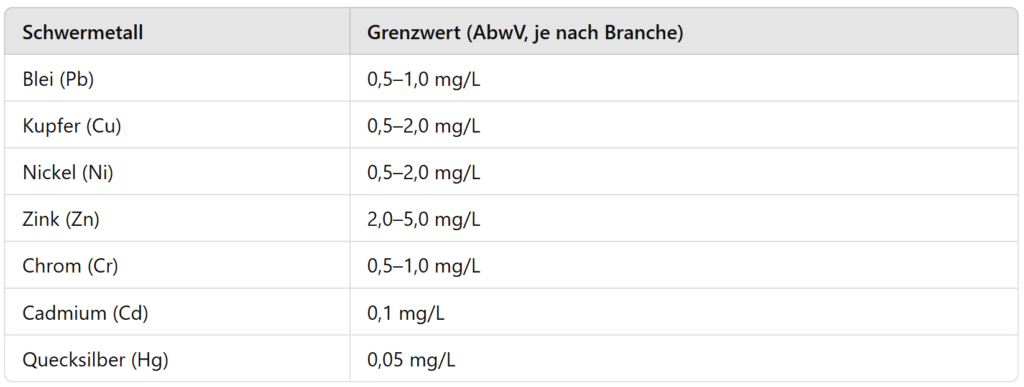
Limit values for heavy metals in industrial wastewater
The discharge of heavy metals into bodies of water or public wastewater systems is strictly regulated. The Wastewater Ordinance (AbwV) sets industry-specific limits that must be adhered to in order to minimize environmental pollution.
Process for removing heavy metals from water and wastewater
The choice of treatment technology depends on the concentration, the type of heavy metal and the water quality requirements. CP systems are often combined with ion exchangers or activated carbon filters.
1. precipitation and flocculation in CP plants
Chemical precipitation is a key process in water and wastewater treatment for the removal of heavy metals. The process is based on the chemical conversion of dissolved heavy metal ions into poorly soluble compounds, which can then be removed from the water by sedimentation, flotation or filtration.
Mechanism of precipitation
Reaction with hydroxide ions (hydroxide precipitation):
- The addition of alkalis such as milk of lime (Ca(OH)₂) or caustic soda (NaOH) leads to an increase in the pH value, causing heavy metal ions to precipitate in the form of sparingly soluble metal hydroxides.
- This produces metal hydroxide precipitates, which can be separated from the water due to their low solubility.
Reaction with sulphides (sulphide precipitation):
- For heavy metals with particularly low solubility as sulphide (e.g. mercury, lead), sulphide ions are used, typically from sodium sulphide (Na₂S).
- Sulphides have the advantage that they form stable precipitates even at slightly acidic pH values.
Precipitation with complexing agents:
- In cases where heavy metals are present in stable complexes (e.g. due to chelating agents such as EDTA), special reagents may be required to break down the complexes before precipitation takes place.
Factors influencing the precipitation
The effectiveness of precipitation depends on various chemical and physical factors that must be carefully controlled:
pH value:
- The pH value is the most important parameter, as the solubility of most heavy metal hydroxides is strongly dependent on the pH value.
- Examples:
- Zinc hydroxide precipitates optimally at pH 9-10.
- Iron(III) hydroxide precipitates at a pH value of 6-8.
- Precise control of the pH value is necessary, as other metals (e.g. aluminum) can become soluble again if the pH value is too high.
Concentration of the reagents:
- A sufficient amount of precipitant is required to bind all heavy metal ions. However, overdosing can lead to increased operating costs and additional chemical consumption.
Temperature:
- The reaction rate and the solubility of the precipitation products are temperature-dependent. Higher temperatures generally promote the reaction rate.
Stirring intensity and contact time:
- Good mixing ensures intensive contact between the reagents and the heavy metal ions.
- The contact time must be long enough for the chemical reactions to take place completely.
Interference from other ions:
- Anions such as chloride or sulphate can increase the solubility of certain heavy metal compounds and thus reduce the efficiency of precipitation.
- In such cases, additional precipitation reagents or pre-treatment steps are required.
Flocculation: stabilization and separation of precipitation products
After precipitation, the precipitation products produced often remain dispersed in the water as fine particles. Flocculation is used to combine these particles into larger aggregates (flocs), which are easier to settle or separate.
Mechanism of flocculation
- Coagulation:
- By adding coagulants such as iron(III) or aluminum salts, the electrostatic repulsive forces between the particles are reduced, which facilitates their agglomeration.
- Flocculation:
- Flocculants such as polymers (e.g. polyacrylamides) increase particle formation by binding the flocs together through bridging.
Limits of precipitation and flocculation
Complexed heavy metals:
- Heavy metals that are present in stable organic or inorganic complexes are difficult to precipitate. Prior splitting of the complexes (e.g. by oxidation or reduction) is necessary.
Residual concentrations:
- Die chemische Fällung erreicht häufig nicht die extrem niedrigen Restkonzentrationen (< 0,01 mg/l) , die in manchen Industrien erforderlich sind. In solchen Fällen sind ergänzende Verfahren wie Adsorption oder Ionenaustausch erforderlich.
Sludge production:
- Precipitation generates large quantities of precipitation sludge, which must be treated and disposed of as hazardous waste. The disposal costs can be considerable.
Chromium(VI):
- Heavy metals such as chromium(VI) must be reduced to the less toxic form chromium(III) before precipitation, for example by adding sodium bisulphite.

Photo: Our CP system ALMA CHEM MCW with precipitation and flocculation, sludge dewatering and downstream multi-layer filter and ion exchanger (if required)
2. ion exchange
Ion exchange is a physico-chemical process in which dissolved ions in water are replaced by ions in a solid ion exchange resin. The process is based on the specific binding of cations or anions to active groups in a resin material. Due to the high selectivity of the resins, heavy metals can be removed even from highly diluted solutions.
Mechanism of ion exchange
- Cation exchange:
- Heavy metal cations such as Cu²⁺, Zn²⁺ or Pb²⁺ are replaced by H⁺ ions (hydrogen-based resins) or Na⁺ ions (sodium-based resins).
- The heavy metal ion is bound to the resin matrix and removed from the water.
- Anion exchange:
- Negatively charged heavy metal complexes, such as chromates (CrO₄²-), are replaced by OH- ions.
Types of ion exchangers
Strong cation exchangers:
- Effective in removing heavy metals such as copper, nickel and zinc from acidic solutions.
- Can be used at pH values from 1 to 14.
Weak cation exchangers:
- Effective at medium to high pH values; ideal for slightly acidic to neutral solutions.
Strong anion exchangers:
- Remove negatively charged complexes such as chromates or arsenates.
- Use in alkaline solutions.
Selective resins:
- Developed for specific heavy metals such as mercury, cadmium or chromium.
Factors influencing the ion exchange
ion concentration:
- Ion exchange is particularly efficient at low concentrations, as the resins have a high affinity for heavy metal ions.
pH value:
- Der pH-Wert beeinflusst die Ladung der Schwermetalle und somit ihre Bindung an das Harz. Beispielsweise liegt Eisen bei pH < 3 als Fe³⁺ vor und kann leicht entfernt werden, während es bei höheren pH-Werten zu Hydroxid fällt.
Competition:
- The presence of other cations such as Ca²⁺ or Mg²⁺ can reduce the effectiveness of the ion exchange.
Resin capacity:
- The maximum load of the resin is limited by its specific capacity (equivalent ions per volume).
Regeneration:
- Once the capacity limit has been reached, the resin is regenerated using chemicals such as hydrochloric acid (HCl) or caustic soda (NaOH).
Advantages and limitations of ion exchange
Advantages:
- Very high selectivity, even for low concentrations of heavy metals.
- Regenerable, which reduces operating costs.
- Can be customized for specific metals (e.g. chrome or copper).
Limits:
- Limited capacity at high concentrations.
- Competition from other ions can reduce efficiency.
- Regeneration chemicals generate additional waste water.

Photo: Our ALMA ION ion exchanger system with upstream ALMA FIL AK activated carbon filter
3. activated carbon adsorption
Adsorption using activated carbon filters is based on the physical or chemical adsorption of heavy metal ions or heavy metal complexes on the surface of a porous material such as activated carbon. The large inner surface of the activated carbon is used to bind the dissolved substances from the water.
Mechanism of adsorption
- Physical adsorption:
- Heavy metals are held on the surface of the activated carbon by van der Waals forces or electrostatic interactions.
- Chemisorption:
- Heavy metals form chemical bonds with functional groups on the surface of the activated carbon (e.g. carboxyl or hydroxyl groups).
Types of activated carbon
Powdered activated carbon (PAH):
- Fine powder that is added directly to the water.
- Particularly suitable for batch treatments.
Granular activated carbon (GAK):
- Coarse-grained activated carbon used in filters.
- Long-term and continuous use possible.
Impregnated activated carbon:
- Treated with chemical reagents (e.g. sulphur) to efficiently remove specific heavy metals such as mercury or arsenic.
Factors influencing adsorption
concentration of heavy metals:
- Higher concentrations lead to better utilization of the adsorption capacity, but also to faster saturation.
pH value:
- The pH value influences the charge of the heavy metal ions and the surface charge of the activated carbon.
- Example:
- At a low pH value, heavy metals such as Pb²⁺ are positively charged and bind well to the negatively charged activated carbon.
Temperature:
- Adsorption processes are usually exothermic, so increasing the temperature can reduce efficiency.
Pore structure:
- The size and distribution of the pores in the activated carbon influence the binding capacity.
Optimization of adsorption
- Pre-treatment of the water:
- Removal of suspended solids and organic compounds improves effectiveness.
- Use of impregnated activated carbon:
- Specially treated activated carbon is much more effective for heavy metals such as arsenic and mercury.
- Regeneration:
- The saturation of the activated carbon can be reversed by thermal or chemical processes, which reduces operating costs.
Advantages and limitations of activated carbon adsorption
Advantages:
- High efficiency with low heavy metal concentrations.
- Versatile, can also be used for other pollutants such as organic compounds.
- Can be impregnated for specific heavy metals.
Limits:
- Limited capacity of the adsorption materials.
- Regeneration is energy and chemical intensive.
- Less suitable for high concentrations of heavy metals.

Photo: Our ALMA FIL AK activated carbon filters with upstream ALMA FIL multi-layer filter
Challenges in heavy metal reduction
Residual concentrations:
- Heavy metals often have to be reduced to very low concentrations, which is associated with rising costs.
- Zielwerte: z. B. Chrom < 0,05 mg/L, Nickel < 0,5 mg/L.
Sludge management:
- Processes such as chemical precipitation generate large quantities of sludge, the disposal of which is cost-intensive.
Complexing agent:
- Organic substances such as EDTA or citrates can stabilize heavy metals, which makes it more difficult to remove them.
Combination of technologies:
- In practice, several processes are often combined, e.g. precipitation and adsorption, in order to achieve optimum results.
Conclusion
Heavy metal reduction is an essential part of industrial water and wastewater treatment. Different technologies are used depending on the specific requirements and composition of the wastewater. Precipitation and flocculation in CP plants and ion exchange are suitable for high metal concentrations, while adsorption using activated carbon filters dominates in fine purification. Careful process design and monitoring are crucial in order to comply with legal requirements and protect the environment in the long term.
For further information on our products, please feel free to contact us at any time!








