The oxidation is a fundamental chemical process that plays a crucial role in industrial water and wastewater treatment. It is used to remove pollutants, convert toxic substances into more harmless compounds and disinfect water. A variety of pollutants such as organic substances, metals and microorganisms can be treated through the targeted use of different oxidation processes.
This article examines oxidation in all its facets. From the chemical principles and the technologies used to the specific applications in practice - this explanation offers a comprehensive transfer of knowledge for engineers and practitioners.
Table of contents
Basics of oxidation
Definition and principle
Oxidation is a chemical reaction in which electrons are transferred from one substance (electron donor) to another substance (electron acceptor). Oxygen (O₂) is often the electron acceptor, but other oxidizing agents such as chlorine, ozone or hydrogen peroxide can also take on this role.
Basic reaction:
Reducing agent (donor)+oxidizing agent (acceptor)→oxidized substance+reduced substance
Important terms in oxidation
- Oxidizing agents: Substances that accept electrons and thereby enable the oxidation of another substance (e.g. ozone, chlorine, hydrogen peroxide).
- Reducing agents: Substances that release electrons and are oxidized (e.g. organic pollutants, metals, ammonium).
- Redox potential: A measure of the oxidizing power of a substance, measured in volts (V). The higher the redox potential, the stronger the oxidizing agent.
Types of oxidation in water technology
There are different types of oxidation that are used depending on the objective and wastewater composition.
1. direct oxidation
- In direct oxidation, the oxidizing agent reacts directly with the pollutant.
- Examples:
- Oxidation of iron (Fe²⁺) to iron hydroxide (Fe(OH)₃).
- Oxidation of hydrogen sulphide (H₂S) to sulphuric acid (H₂SO₄).
2. indirect oxidation
- Reactive intermediate products such as hydroxyl radicals (-OH) are formed here, which then attack the pollutants.
- Examples:
- Formation of hydroxyl radicals from ozone (O₃) or hydrogen peroxide (H₂O₂).
Oxidizing agents in industrial water and wastewater technology
1. ozone (O₃)
- Properties: Very strong oxidizing agent with a redox potential of +2.07 V.
- Areas of application:
- Degradation of organic pollutants (e.g. pharmaceutical residues).
- Disinfection.
- Removal of color and odor.
- Advantages: No residues, as ozone decomposes into oxygen.
2. chlorine (Cl₂)
- Properties: Strong oxidizing agent that forms hypochlorous acid (HOCl) in aqueous solution.
- Areas of application:
- Disinfection.
- Oxidation of ammonium (NH₄⁺) to nitrogen gas (N₂).
- Removal of iron and manganese.
- Challenges: Formation of chlorine-containing by-products such as trihalomethanes (THMs).
3. hydrogen peroxide (H₂O₂)
- Properties: Strong oxidizing power, often used in combination with UV reactors or catalysts.
- Areas of application:
- Treatment of heavily contaminated wastewater.
- Removal of cyanides or phenols.
4. potassium permanganate (KMnO₄)
- Properties: Strong oxidizing agent for the oxidation of metals and sulphur compounds.
- Areas of application:
- Removal of iron and manganese.
- Oxidation of hydrogen sulphide.
Oxidation technologies and processes
1. classic oxidation systems
- Use of ozone, chlorine or hydrogen peroxide in reactors.
- Components:
- Dosing systems for the oxidizing agent.
- reaction tanks or pressure reactors.

Photo: Our reactor for wet-chemical oxidation ALMA BHU UXI using ozone or according to Fenton
2. UV-assisted oxidation (UV/H₂O₂)
- Combination of UV reactors with hydrogen peroxide to form hydroxyl radicals.
- Application:
- Degradation of organic micropollutants such as pesticides and pharmaceuticals.
- Advantages: No formation of by-products such as chlorine compounds.
3. wet oxidation
- Use of high temperatures (150-350 °C) and pressures (50-250 bar) to oxidize pollutants.
- Application:
- Treatment of heavily contaminated wastewater from the chemical industry.
4. electrochemical oxidation
- Direct oxidation at an anode or indirect oxidation by electrochemically generated reagents such as ozone.
- Advantages: No additional use of chemicals.
- Application:
- Treatment of highly concentrated wastewater.
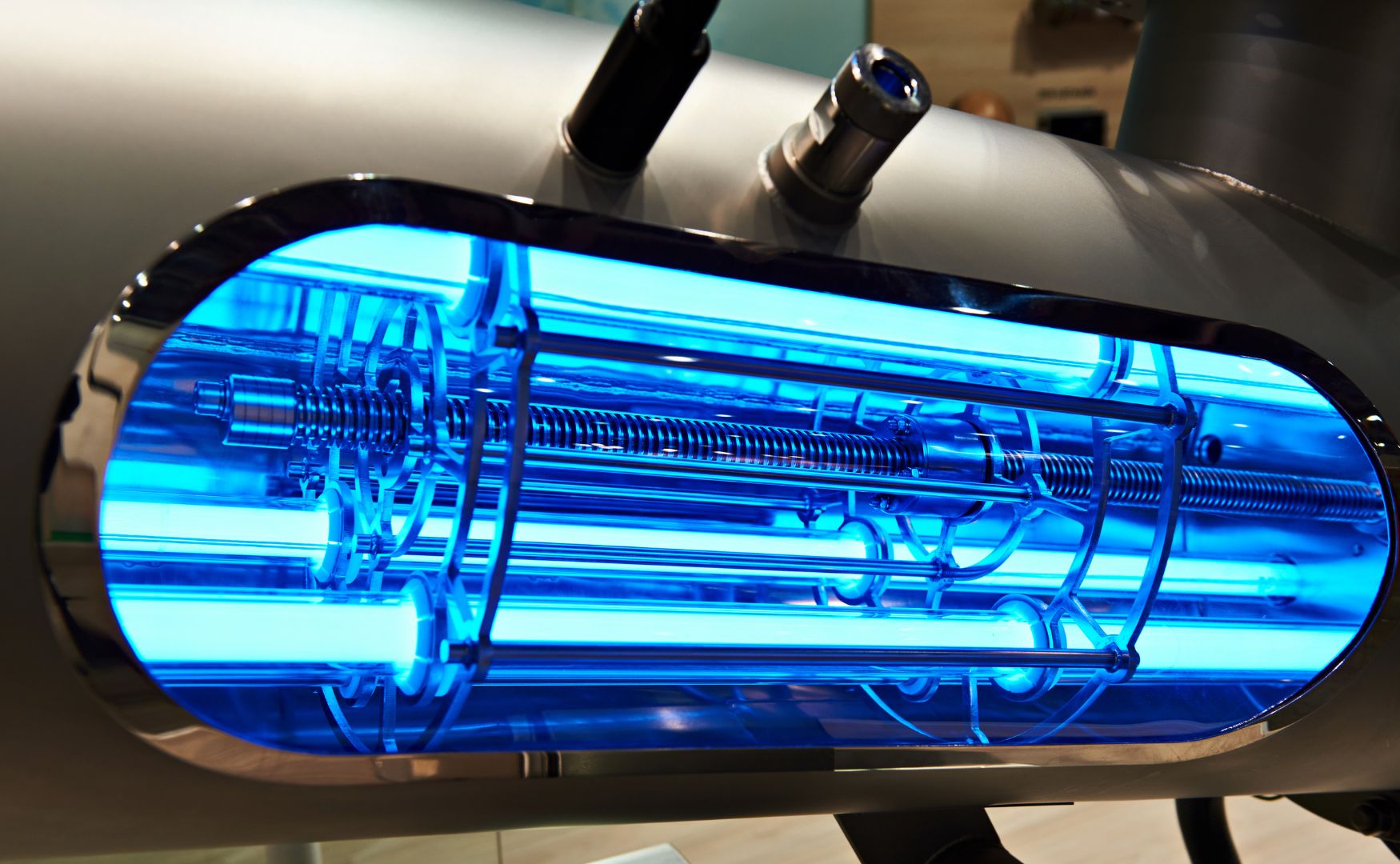
Photo: UV reactor with defined UV spectrum for the formation of highly radical hydroxyl radicals of the ALMA OXI UV
Applications of oxidation
1. removal of micropollutants
- Oxidation of endocrine disruptors, drug residues and pesticides in wastewater treatment plants.
2. disinfection
- Killing of microorganisms in drinking water and cooling circuits.
3. metal oxidation
- Oxidation of iron (Fe²⁺) and manganese (Mn²⁺) to insoluble oxides, which can be removed by filtration.
4. color reduction
- Removal of dyes from wastewater from the textile and paper industry.
5. odour neutralization
- Oxidation of hydrogen sulphide and other odor-forming substances.
The challenges of oxidation
Corrosion:
- Highly reactive oxidizing agents attack system materials.
- Solution: Use corrosion-resistant materials such as PTFE or V4A stainless steel.
Formation of by-products:
- Oxidation processes can generate undesirable by-products (e.g. bromate during ozone treatment).
- Solution: Optimization of dosing and reaction conditions.
Energy requirement:
- Processes such as ozone generation or UV oxidation are energy-intensive.
- Solution: Integration of energy efficiency measures.
Conclusion
Oxidation is an essential process in water and wastewater treatment, covering numerous applications from pollutant removal to disinfection. With the targeted selection of oxidants and technologies, specific challenges can be overcome efficiently. Thanks to innovations such as UV-assisted oxidation and electrochemical processes, oxidation will continue to offer enormous potential for sustainable and effective water management in the future.
For further information on our products, please feel free to contact us at any time!