Organic compounds are an extensive class of chemical substances consisting of carbon (C) and usually hydrogen (H), often combined with oxygen (O), nitrogen (N), sulphur (S), phosphorus (P) or halogens. They play a central role in industrial water and wastewater treatment, as they occur in the form of pollutants, nutrients, energy sources and even operating materials. Understanding their chemical properties, their behavior in water and their interactions with treatment processes is crucial in order to develop efficient water and wastewater treatment systems.
Table of contents
Properties and classification of organic compounds
Organic compounds can be divided into different categories depending on their chemical structure, origin or function:
1. hydrocarbons
- Consist exclusively of carbon and hydrogen atoms.
- Subdivision into:
- Aliphatic hydrocarbons (e.g. methane, hexane): Single chains or branched chains.
- Aromatic hydrocarbons (e.g. benzene, toluene): Ring structures with delocalized electrons.
- Relevance: Common components of industrial wastewater from the petrochemical industry.
2. halogenated organic compounds
- Hydrocarbons containing halogens (Cl, Br, F).
- Examples: Trichloroethylene, dichloromethane.
- Relevance: Highly persistent and toxic, poorly biodegradable.
3. organic acids
- Carboxyl groups (-COOH) give these compounds acidic properties.
- Examples: Acetic acid, citric acid.
- Relevance: Influence the pH value and the precipitation processes in water treatment systems.
4. organic nitrogen compounds
- Contain amino groups (-NH₂) or other nitrogen compounds.
- Examples: Amines, urea.
- Relevance: Can increase nitrogen inputs into water bodies and contribute to eutrophication.
5. organic phosphorus compounds
- Contain phosphorus in organic binding.
- Examples: Pesticides, phospholipids.
- Relevance: Contribute to nutrient pollution.
6. biopolymers and natural substances
- Carbohydrates, fats, proteins, lignin and humic substances.
- Relevance: Common in municipal and agricultural wastewater.
7. synthetic organic compounds
- Plastics, surfactants, pharmaceutical residues, plasticizers (e.g. BPA, PAH).
- Relevance: Increasing problem in water treatment due to their persistence and toxic effect.
Sources of organic compounds in water and wastewater
Industrial processes
- Petrochemical industry: hydrocarbons, aromatics, solvents.
- Chemical industry: Halogenated compounds, surfactants, plastics.
- Food industry: organic acids, fats, proteins.
Household wastewater
- Cleaning agents, personal care products, pharmaceutical residues.
- Organic compounds such as surfactants and fragrances enter wastewater treatment plants via wastewater.
Agriculture
- Input from pesticides, herbicides and animal excrement.
- Degradation products of organic substances accumulate in surface and groundwater.
Natural sources
- Decomposition of organic matter such as plant residues and humic substances.
- Dissolved organic carbon (DOC) from natural decomposition processes.
Relevance of organic compounds in water and wastewater treatment
1. pollutants and environmental pollution
Many organic compounds are toxic, carcinogenic or mutagenic and difficult to biodegrade. These include
Halogenated compounds such as trichloroethylene and PCBs, which are persistent and bioaccumulative.
- Danger: Toxicity to aquatic organisms, potential carcinogenicity.
- Grenzwert: AOX im Trinkwasser < 0,1 mg/L (Deutschland).
PAHs (polycyclic aromatic hydrocarbons), e.g. benzo[a]pyrene, which originate from combustion processes.
- Danger: Carcinogenicity and damage to ecosystems.
- Grenzwert: PAK (Benzo[a]pyren) im Trinkwasser < 0,01 µg/L (EU).
Drug residues, e.g. diclofenac and antibiotics, which are biologically active and difficult to break down.
- Danger: Development of resistance in microorganisms, disruption of hormone systems.
- Richtwert: Empfohlen < 100 ng/L (keine gesetzlichen Grenzwerte).
Influences on ecosystems:
- Even low concentrations can have a toxic effect on fish and amphibians, impairing reproduction and growth.
- Persistent substances accumulate in sediments and remain active for decades.
Challenges and requirements:
- Conventional wastewater treatment plants cannot completely remove many of these compounds. Persistent substances require specialized processes such as adsorption or oxidation.
- Grenzwertbeispiele: EU-Wasserrahmenrichtlinie: Benzo[a]pyren in Oberflächengewässern < 0,03 µg/L.
2. nutrients and eutrophication
Organic compounds containing nitrogen and phosphorus are important nutrients. However, in high concentrations they promote eutrophication, which significantly disturbs the balance of water bodies.
Nitrogen compounds (e.g. ammonium, urea):
- Properties: Rapidly bioavailable, promotes the growth of algae.
- Consequences: Algal blooms (phytoplankton) lead to oxygen deficiency (hypoxia) and damage aquatic organisms.
- Grenzwert: Ammonium im Trinkwasser < 0,5 mg/L (Deutschland).
Phosphorus compounds (e.g. orthophosphates, phospholipids):
- Properties: Even small quantities can trigger eutrophying effects.
- Consequences: Formation of toxic blue-green algae and deterioration in water quality.
- Grenzwerte: Gesamtphosphor in Kläranlagen < 1 mg/L (EU), Phosphat im Trinkwasser < 6,7 mg/L (Deutschland).
Influences on wastewater treatment:
- High concentrations of organic nutrients, e.g. from agriculture or industry, overwhelm biological treatment processes.
- Excess nutrients are removed by nitrification/denitrification (nitrogen) or chemical precipitation (phosphorus).
Process for removing organic compounds
The removal of organic compounds from water and wastewater is a key aspect of industrial water treatment. Due to the different properties of organic compounds - from easily degradable to highly persistent - various physical, chemical and biological processes are used. These are often combined to ensure maximum efficiency.
1. biological processes
Principle: Biological processes use microorganisms to break down or convert organic compounds. The microorganisms oxidize organic substances to CO₂ and water or reduce them to methane and other intermediate products.
Examples of biological processes:
- Microorganisms oxidize organic substances under aerobic conditions.
- Application: Wastewater treatment plants for municipal and industrial wastewater.
- Process: Oxygen is introduced into the aeration tank by aerators to promote the activity of the bacteria.
- Typical decomposition products: CO₂ and biomass (sewage sludge).
Anaerobic wastewater treatment:
- In oxygen-free reactors, microorganisms convert organic compounds into biogas (methane and CO₂).
- Application: Suitable for highly contaminated wastewater (e.g. from the food industry).
- Process: Organic substrates are broken down in stages by hydrolytic, acidogenic, acetogenic and methanogenic microorganisms.
Advantages of biological processes:
- Cost-effective, as no expensive chemicals are required.
- Sustainable, especially through biogas production.
Disadvantages of biological processes:
- Not suitable for poorly degradable (refractory) organic compounds such as PAHs or halogenated hydrocarbons.
- Sensitive to temperature fluctuations and toxic substances.

2. chemical oxidation
Principle: Chemical oxidation processes destroy organic compounds by generating reactive species such as hydroxyl radicals (OH-), which oxidize organic molecules and break them down into CO₂, H₂O and smaller molecules.
Typical oxidizing agents:
- Ozone (O₃): Strong oxidizing agent that is used directly or in combination with hydrogen peroxide.
- Hydrogen peroxide (H₂O₂): Often used in combination with UV light or ozone.
- Chlorine: Used for disinfection and oxidation, but with the risk of forming chlorinated by-products.
Advanced Oxidation Processes (AOPs):
- Mechanism: Combination of oxidizing agents with UV light or catalysts to generate hydroxyl radicals.
- Application: Degradation of persistent pollutants such as halogenated hydrocarbons, pharmaceutical residues and polycyclic aromatic hydrocarbons (PAHs).
Advantages of chemical oxidation:
- Suitable for poorly degradable organic compounds.
- Faster degradation compared to biological processes.
Disadvantages of chemical oxidation:
- High operating costs due to the consumption of chemicals and energy.
- Formation of by-products
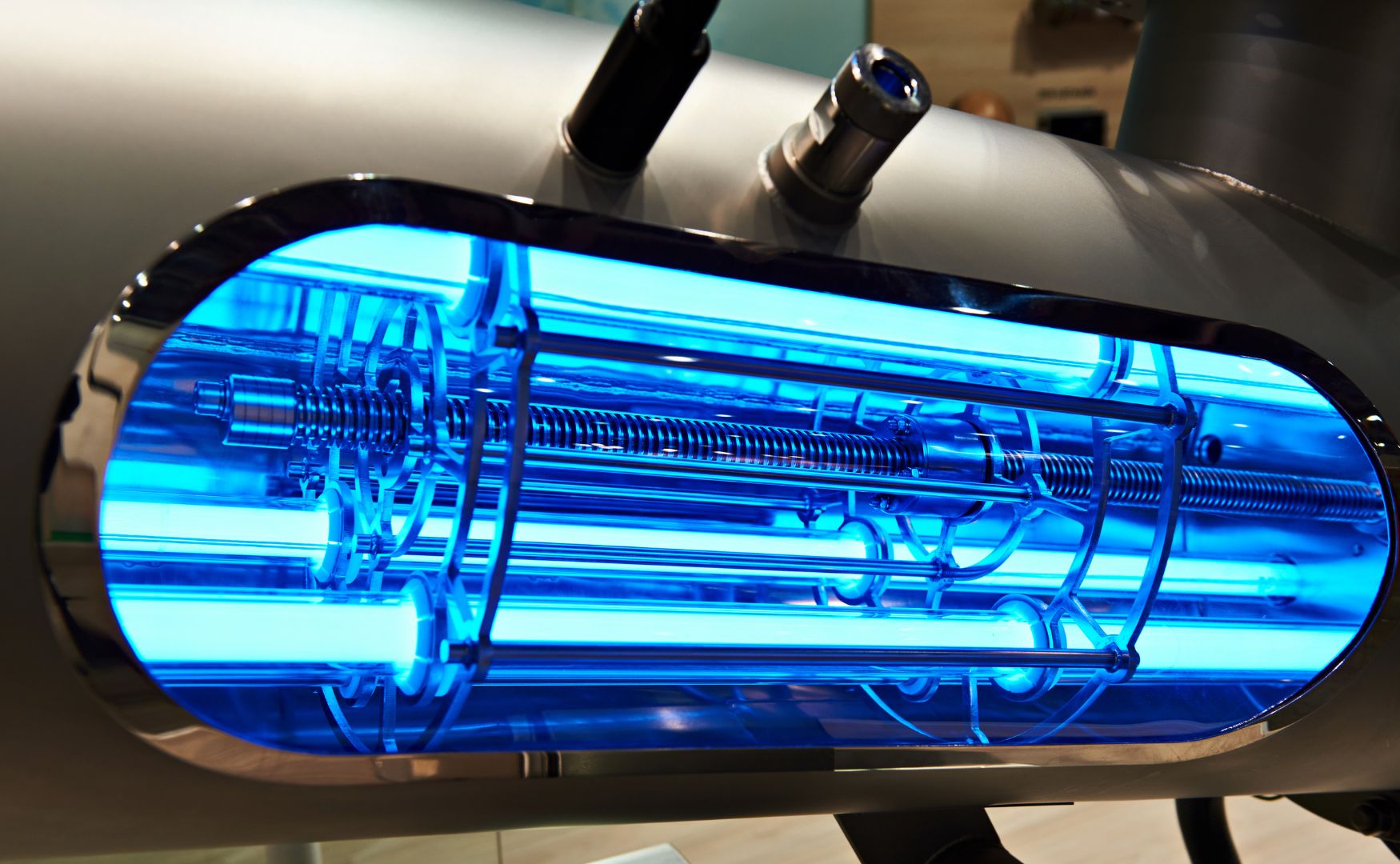
Photo: Our UV oxidation system ALMA OXI UV for the removal of persistent organic compounds and micropollutants
3. adsorption
Principle: Adsorption processes bind organic compounds to porous materials such as activated carbon, which have a large specific surface area and a high affinity for organic molecules.
Procedure:
- The contaminated water is passed through adsorption filters, whereby the organic compounds bind to the surface of the activated carbon.
- Once saturation is reached, the activated carbon is regenerated or replaced.
Applications:
- Drinking water treatment: removal of micropollutants and flavorings.
- Wastewater treatment: Removal of residues after biological treatment.
Advantages of adsorption:
- High efficiency in the removal of trace substances and poorly degradable compounds.
- Relatively simple integration into existing systems.
Disadvantages of adsorption:
- Limited absorption capacity of the adsorbents.
- High costs for regeneration or disposal of the saturated activated carbon.

Photo: Our ALMA FIL AK activated carbon filters with upstream multi-layer filters
5. precipitation and flotation
Principle: Chemical precipitation uses reagents that form poorly soluble compounds with organic pollutants, which can then be removed by flotation or sedimentation.
Procedure:
- Precipitants such as iron or aluminum salts are added to the water to bind organic substances.
- In flotation, the flocs formed are driven to the surface by air bubbles and skimmed off.
Applications:
- Treatment of wastewater with high organic loads, e.g. from the food industry or refineries.
Advantages of precipitation and flotation:
- Fast and effective for heavily contaminated wastewater.
- Reduction of COD and BOD.
Disadvantages of precipitation and flotation:
- High chemical consumption.
- Formation of large quantities of sludge that must be disposed of.

Photo: Our ALMA NeoDAF flotation system with precipitation and flocculation to reduce organic compounds
Monitoring and analysis of organic compounds
Sum parameters:
- Chemical oxygen demand (COD): Measure of the quantity of oxidizable organic compounds.
- Biochemical oxygen demand (BOD5): Proportion of biodegradable organic compounds.
- TOC (Total Organic Carbon): Total content of organic carbon.
Specific analysis:
- Gas chromatography (GC) and liquid chromatography (HPLC) for the identification of specific compounds.
- Mass spectrometry (MS) for the analysis of trace substances.
Online monitoring:
- Continuous monitoring of COD and TOC in wastewater streams.
Conclusion
Organic compounds pose a complex challenge in industrial water and wastewater treatment. Their effective removal requires a deep understanding of their chemical properties and the targeted interaction of modern technologies. By using combined processes and precise monitoring, environmental regulations can be met and economical and sustainable solutions realized.
For further information on our products, please feel free to contact us at any time!