Chromium reduction is an essential chemical process in industrial wastewater treatment that aims to convert the highly toxic chromium(VI) (hexavalent chromium) into the less toxic and easier to remove form chromium(III) (trivalent chromium). Chromium(VI) is water-soluble, highly oxidizing and is considered carcinogenic, which makes it a significant environmental and health risk. Reduction to chromium(III) is a critical step in efficiently removing chromium from industrial wastewater and complying with the legal limits for discharge into water bodies.
Table of contents
Technical background
Chromium occurs in two main oxidation states: Chromium(III) and Chromium(VI). While chromium(III) is a relatively harmless form that even occurs as an essential trace element in some biological processes, chromium(VI) is highly toxic, extremely mobile in aqueous systems and more difficult to remove. As chromium(VI) remains soluble in acidic to neutral pH ranges, it poses a particular challenge in wastewater treatment.
The reduction process from chromium(VI) to chromium(III) is carried out by adding reducing agents. These reducing agents release electrons that convert the hexavalent chromium into trivalent chromium. The resulting chromium(III) is then removed by chemical precipitation from the water.
Chemical reaction
The reduction of chromium(VI) to chromium(III) can be carried out with various reducing agents. Common reducing agents are
Sodium bisulfite (NaHSO₃)
In this reaction, the hexavalent chromium (dichromate ion) is reduced to trivalent chromium, while the sulphite ion is oxidized.
Sulphur dioxide (SO₂)
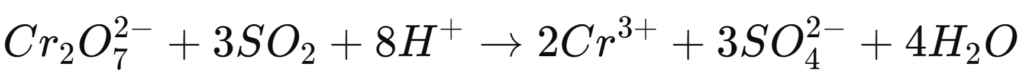
Sulphur dioxide is another frequently used reducing agent, which is also used to reduce chromium(VI) to chromium(III).
Iron(II) sulphate (FeSO₄)
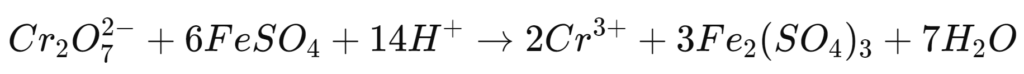
In this reaction, the iron(II) ion acts as an electron donor that reduces chromium(VI).
After reduction, the relatively harmless chromium(III) is produced, which is precipitated as chromium hydroxide [Cr(OH)₃] in a further stage. This is done by adding alkaline solutions such as sodium hydroxide (NaOH) or milk of lime (Ca(OH)₂) to raise the pH value to an alkaline range (pH 8-9).
Chromium reduction process steps
1st reduction step
The process of chromium reduction begins with the addition of the reducing agent to the wastewater containing chromium(VI). Under acidic conditions (pH 2-3), the chromium(VI) reacts with the reducing agent and is converted into the trivalent form chromium(III). pH control is crucial to ensure that the reduction is complete.
2. pH adjustment and precipitation
After reduction, the pH value of the wastewater is raised to alkaline values in order to precipitate chromium(III) as chromium hydroxide [Cr(OH)₃]. This precipitation process takes place optimally in the pH range between 8 and 9, as chromium(III) is least soluble in this range. The solids formed can then be removed by sedimentation or filtration be removed.
3. flocculation and sedimentation
In order to efficiently separate the resulting chromium hydroxide particles, flocculants such as polyelectrolytes are added to aggregate the solids into larger flocs. These flocs sink to the bottom of a sedimentation tank and can be removed as chromium sludge.

Photo: CP system ALMA CHEM MCW for the removal of heavy metals, AOX, hydrocarbons, cyanide and chromium (using sodium bisulphite)
4. sludge treatment
The separated chromium sludge contains the separated chromium(III) in the form of chromium hydroxide. This sludge must be dewatered and then disposed of properly so as not to pollute the environment.
5. activated carbon filtration & ion exchange
In some cases, especially with very low permissible limit values for chromium, additional cleaning steps such as activated carbon filtration or ion exchangers can be used to further reduce the residual concentrations of chromium in the water.

Photo: ALMA ION ion exchanger for the removal of specific pollutants such as chromium
Areas of application for chromium reduction
Chromium reduction is used in various industries that produce wastewater containing chromium(VI):
Electroplating industry
In the electroplating industry, chromium(VI) is used for chrome plating of metals and for surface treatments. Chromium reduction is an indispensable process for treating chromium(VI)-containing wastewater to safe levels before it can be discharged or recycled.Leather and textile industry
In the leather tanning and textile industries, chromium(VI) is used in some processes for dyeing or surface treatment. The wastewater from these industries must be thoroughly treated to convert chromium(VI) into the less toxic form chromium(III).Chemical industry
Chromium(VI) is used as an oxidizing agent in chemical synthesis and the production of pigments and dyes. Waste water from these processes often contains chromium(VI), which must be treated by reduction and subsequent precipitation.Glass and ceramics industry
Chromium(VI) is used in glass coloring and ceramic production. Here too, waste water is produced that must be treated before being discharged into the environment.
Advantages of chromium reduction
Efficient removal of chromium(VI): The reduction process is a proven method for safely converting the highly toxic chromium(VI) into the harmless form chromium(III), thus significantly reducing environmental and health hazards.
High flexibility: The process can be applied to various industrial wastewaters and offers a reliable solution for the treatment of heavy metal waste, regardless of the type of industry.
Compliance with environmental standards: With chromium reduction, companies can ensure that they adhere to the strict legal limits for chromium discharges into bodies of water and maintain their compliance.
The challenges of chromium reduction
High chemical consumption: The reduction and subsequent precipitation requires the use of reducing agents and pH correctors, which leads to ongoing operating costs.
Sludge treatment: The precipitation of chromium(III) leads to the formation of chromium sludge, which must be dewatered and disposed of in an environmentally friendly manner. This places additional demands on wastewater and sludge treatment.
Precise pH control: The reduction and precipitation processes require precise pH control to ensure complete reduction and precipitation. Deviations in pH can lead to incomplete chromium reduction or inefficient precipitation.
Conclusion
Chromium reduction is an essential process in industrial wastewater treatment in order to safely convert the toxic and environmentally harmful chromium(VI) into the less toxic chromium(III) and then remove it from the wastewater. With the right pH control, the choice of a suitable reducing agent and subsequent precipitation, the chromium load in the wastewater can be effectively reduced. The ALMA CHEM MCW CP system offers customized solutions for chromium reduction and treatment that enable environmentally friendly and legally compliant wastewater disposal.








