The wet oxidation is an effective method for treating heavily contaminated wastewater that is not accessible to conventional processes. In addition to classic wet oxidation, there are further developments such as chemical oxidation and UV-assisted wet oxidationwhich are tailored to specific requirements and pollutant profiles. These technologies extend the range of oxidation processes that can be used and improve efficiency and applicability.
In this article, the basics, variants and fields of application of wet oxidation, chemical oxidation and UV-assisted wet oxidation are explained in detail.
Table of contents
Chemical principles of wet oxidation
Wet oxidation is based on the oxidation of organic and inorganic pollutants in an aqueous phase at elevated temperatures and pressures. In this process, molecules are broken down into smaller, less harmful components by oxygen (O₂) or other oxidizing agents.
Reaction mechanisms:
Radical formation:
- Oxygen molecules use thermal energy to form radicals (-OH), which are highly reactive.
- These radicals attack the organic pollutants and break them down into harmless components.
Oxidation of organic compounds:
- The organic substances are converted into carbon dioxide and water.
Destruction of toxic inorganic substances:
- Ammonium (NH₄⁺) is oxidized to nitrogen (N₂).
- Sulphur compounds (e.g. H₂S) are converted into sulphates (SO₄²-).
Further developments in wet oxidation
1. chemical oxidation
Chemical oxidation extends classic wet oxidation by using strong oxidizing agents that have an additional oxidative effect.
Oxidizing agents used:
Hydrogen peroxide (H₂O₂):
- Decomposes into hydroxyl radicals (-OH), which have a high oxidizing power.
Ozone (O₃):
- Very strong oxidizing agent that breaks down pollutants directly or via the formation of radicals.
Sodium hypochlorite (NaOCl):
- Effective for destroying organic pollutants, especially in alkaline media.
Areas of application:
- Treatment of wastewater containing toxic organic substances such as phenols or halogenated hydrocarbons.
- Destruction of water contaminated with micro-pollutants from the chemical industry.
Advantages:
- Supplements thermal oxidation with chemical reaction pathways.
- Increases oxidation efficiency at lower temperatures and pressures.
Disadvantages:
- Costs for the procurement and handling of oxidizing agents.
- Potential by-products such as halogenated compounds.

Photo: Our reactor for wet-chemical oxidation ALMA BHU UXI using ozone or according to Fenton
2. UV-assisted wet oxidation
UV-assisted wet oxidation combines thermal or chemical oxidation with UV radiation. UV radiation supports the formation of highly reactive radicals and increases the degradation rate of pollutants.
Functional principle:
- UV rays with a wavelength of 254 nm or lower break chemical bonds and generate hydroxyl radicals (-OH).
- These radicals attack organic pollutants and oxidize them to carbon dioxide, water and inorganic residues.
Combination with oxidizing agents:
- Hydrogen peroxide is split into two hydroxyl radicals by UV rays.
Areas of application:
- Degradation of stubborn organic compounds such as drug residues, pesticides or aromatic hydrocarbons.
- Treatment of wastewater in the pharmaceutical and agrochemical industries.
Advantages:
- Highly efficient pollutant removal.
- Reduces the need for high temperatures and pressures.
Disadvantages:
- Requires UV-resistant reactor materials.
- High energy consumption of the UV lamps.
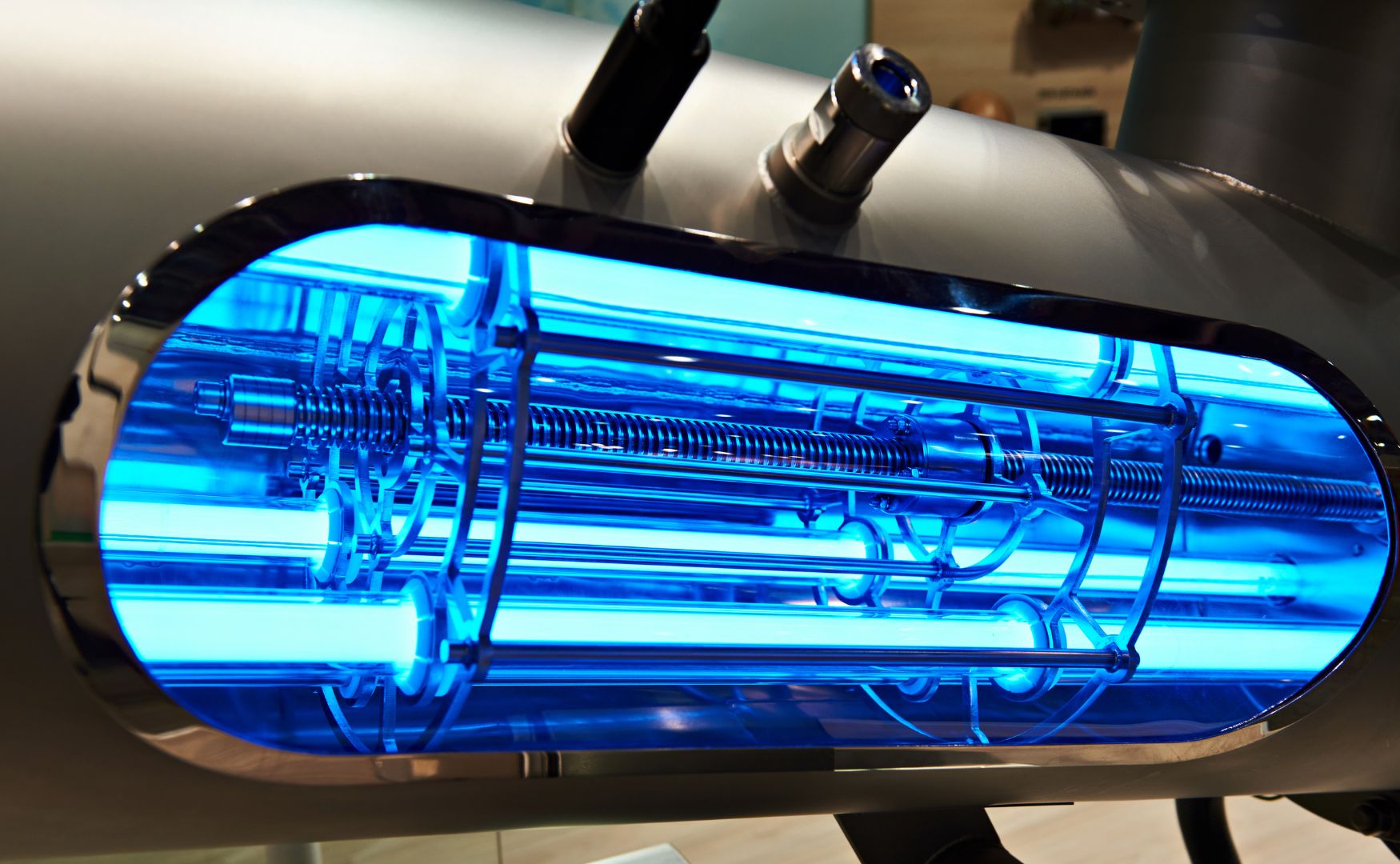
Photo: UV reactor with defined UV spectrum for the formation of highly radical hydroxyl radicals of the ALMA OXI UV
Challenges and optimization
Heat and energy efficiency:
- The integration of heat recovery systems can significantly reduce energy consumption.
Corrosion:
- Aggressive conditions require highly corrosion-resistant materials such as titanium or Hastelloy.
By-products:
- Chemical oxidation processes can generate toxic by-products that require further treatment.
Maintenance and operating costs:
- UV lamps must be regularly maintained or replaced.
- High pressure and temperature require robust and high-maintenance systems.
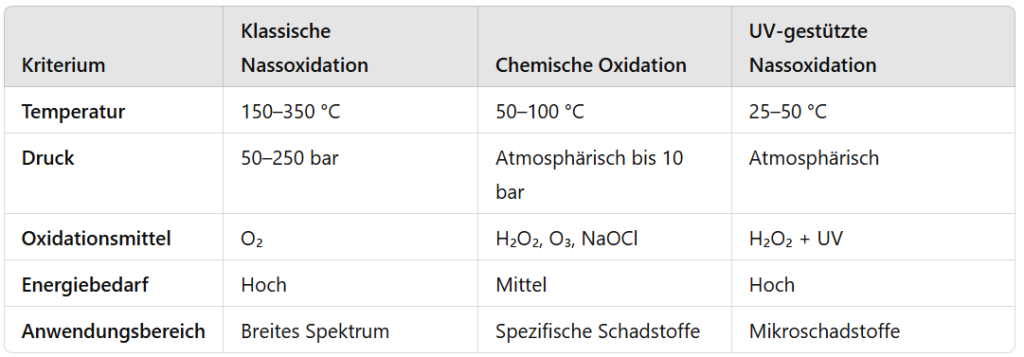
Comparison of the procedures
Conclusion
Wet oxidation is a versatile and powerful process for treating contaminated wastewater. Further developments such as chemical oxidation and UV-assisted wet oxidation extend the range of applications and offer additional efficiency gains. With careful selection of the technology, depending on the specific wastewater parameters, wastewater with high pollutant loads can be treated effectively. In the future, hybrid processes and sustainable energy sources could further improve the economic and ecological efficiency of these processes.
For further information on our products, please feel free to contact us at any time!







