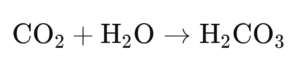Neutralization, a chemical reaction in which acids and bases react to form salts and water, plays a central role in industrial wastewater treatment. Wastewater often has to be neutralized before it can be discharged into the public sewer system. This applies in particular to acidic and alkaline wastewater from various industrial processes. As a rule, the pH value is between 6.5 and 9.5 according to the official discharge requirements.
Table of contents
Basics of neutralization
Lyes such as milk of lime or caustic soda are usually used to neutralize acidic wastewater. Conversely, alkaline wastewater is often neutralized with acids such as hydrochloric acid or sulphuric acid. However, neutralization does not always mean adjusting the pH value to 7, but also adjusting it to specific pH values to precipitate heavy metal hydroxides. A frequent disadvantage of these processes is the increased salt load in the wastewater.
Typical chemicals
- Acids: sulphuric acid (H₂SO₄), hydrochloric acid (HCl), carbonic acid (H₂CO₃)
- Caustic solutions: caustic soda (NaOH), milk of lime (Ca(OH)₂), soda solution (Na₂CO₃)
Procedures and challenges
Neutralization is the most frequently carried out corrective treatment in wastewater treatment. The pH value is brought into a neutral range (pH 6 to 9) by adding acids or bases.
The general reaction equation is
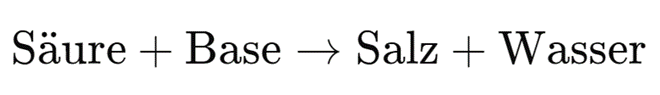
This is done by means of pH value control and dosing of the neutralizing agents. The precise dosing of acid and alkali is carried out by automatic pH value control. Fluctuating wastewater compositions can make this automatic control difficult, as the pH value is based on a logarithmic scale.

Photo: Dosing station for acid and lye installed in a technical room container (System: ALMA Neutra)
Process engineering implementation
The neutralization process technology comprises several steps and equipment that can vary depending on the type and quantity of wastewater.
Homogenization of the wastewater:
If necessary, the wastewater is cleaned of coarse solids. This can be done using mechanical filters and sedimentation tanks. The wastewater is then temporarily stored in large storage tanks and mixed to ensure a homogeneous wastewater composition.
Mixing and dosing:
Acids or alkalis are added in a controlled manner using dosing pumps. It is important to distribute the chemicals evenly in the wastewater. Static mixers, neutralization lines or dynamic agitators are used for this purpose.
pH measurement and control:
The pH value is continuously monitored using pH probes that send data to a control system in real time. This system controls the dosing pumps to ensure precise adjustment of the pH value.
Reaction vessel:
Neutralization takes place in special reaction tanks with agitators and aerators or in neutralization lines. The reaction time is crucial to ensure that the neutralization reaction is complete.
Final inspection:
Before the wastewater is discharged into the public sewer system or watercourse, a final check of the pH value and other parameters is carried out. This check ensures that all legal requirements are met.

Photo: Neutralization section with flow and pH control (system: ALMA Neutra)
Challenges
The neutralization of wastewater poses a number of challenges that require careful planning and implementation:
- Fluctuating wastewater composition: Industrial wastewater can vary greatly in its composition, which requires flexible and adaptable control technology.
- Dosing accuracy: Precise dosing of acids and alkalis is crucial to avoid over- or underdosing, which could lead to environmental problems or inefficient neutralization.
- Safety: Handling strong acids and bases requires special safety precautions to protect personnel and the environment.
Neutralization with CO₂: Sustainable wastewater treatment
An economical and safe process for neutralizing alkaline wastewater is the use of carbon dioxide (CO₂). In contrast to strong acids, carbon dioxide offers a flatter neutralization curve. This facilitates precise control of the pH value and prevents over-acidification of the wastewater. In addition, the addition of CO₂ increases the buffering capacity of the wastewater, resulting in a more stable pH value.
These reactions of CO₂ with the wastewater constituents produce carbonates and hydrogen carbonates, which are more environmentally friendly than the salts of strong acids.

Photo: CO₂ neutralization plant (plant: ALMA Neutra)
Flue gas neutralization: sustainability and CO₂ reduction
A particularly sustainable method of neutralization is the use of CO₂-containing flue gas from boiler systems. This method significantly improves a company's CO₂ footprint by utilizing the flue gas sensibly and economically and using it in the neutralization process.
Process and advantages
- Sustainability: The use of flue gas for neutralization is an environmentally friendly method that reduces a company's CO₂ emissions. The CO₂ contained in the flue gas is not released into the atmosphere, but is used to neutralize wastewater.
- Economic efficiency: By using existing resources, such as flue gas from boilers, the need for externally supplied CO₂ or other neutralizing agents is reduced. This lowers operating costs and improves the overall cost-effectiveness of the process.
- Conversion: The CO₂ in the flue gas reacts with the alkalis in the wastewater and forms stable carbonates and hydrogen carbonates, which are less harmful to the environment.
Chemical reaction
Reaction of CO₂ with water to form carbonic acid.
Plant systems for CO₂ neutralization
There are various plant systems for the effective injection of CO₂ into wastewater:
- Static mixers: Homogeneous distribution of CO₂ through static mixing elements.
- Agitators: Mechanical mixing of the wastewater with CO₂.
- Submersible aerators: Introduction of CO₂ through submersible aeration systems
- External reactors: Separate reactors for CO₂ injection.
All systems are equipped with measuring, control and recording devices to meet legal requirements. The pH value, temperature and wastewater volumes are automatically recorded and saved before being discharged into the sewer system.
Conclusion & summary
The neutralization of wastewater is an essential step in complying with environmental regulations and ensuring the operational stability of wastewater treatment plants. The use of CO₂, especially from flue gas, offers an environmentally friendly and economical alternative to traditional methods using acids and alkalis. Companies that rely on this method not only benefit from an improved CO₂ balance, but also from long-term economic advantages.
For further information and individual advice on wastewater neutralization, please do not hesitate to contact us. You can find out more about our ALMA Neutra neutralization system at the following link.

Photo: Neutralization system with grease separator, pump shaft, stacking container and neutralization system installed in the technical room container (system: ALMA Neutra)