The UV oxidation is an advanced process that combines physical and chemical processes to efficiently remove organic pollutants, micropollutants and pathogenic microorganisms from water and wastewater. This process uses ultraviolet (UV) radiation in conjunction with oxidizing agents such as hydrogen peroxide (H₂O₂) or ozone (O₃) to generate highly reactive hydroxyl radicals (-OH). These radicals have an extremely high oxidation potential and break down pollutants into harmless compounds such as carbon dioxide and water.
This article explains UV oxidation in detail, including the chemical principles, technologies used, applications and practical challenges.
Table of contents
Basics of UV oxidation
UV radiation and its effects
UV radiation is electromagnetic radiation in the wavelength range of 100-400 nm. UV-C radiation (200-280 nm) has the highest energy and is most effective for water treatment. It is used to:
- To break chemical bonds and destroy pollutants directly.
- oxidizing agents such as H₂O₂ or O₃ into reactive species such as hydroxyl radicals (-OH).
Reactive species in UV oxidation
Hydroxyl radicals are the driving force behind UV oxidation. They have an oxidation potential of +2.8 V and react non-selectively with almost all organic compounds.
The hydroxyl radicals attack pollutants and oxidize them to carbon dioxide, water and other harmless compounds.
Design and components of a UV oxidation system
UV oxidation systems consist of various technological components that are tailored to the specific requirements of water or wastewater treatment.
1. UV reactor
- Function: The UV reactor is the heart of the system. This is where the water is irradiated and the hydroxyl radicals are produced.
- Design:
- Tubular or chamber reactors with UV lamps arranged centrally or along the flow.
- Made from UV-resistant materials such as quartz glass or stainless steel.
2. UV lamps
- Types:
- Low-pressure lamps (LP): Low energy consumption, suitable for disinfection.
- Medium-pressure lamps (MP): Higher radiation intensity, ideal for oxidation.
- Service life: 8,000-16,000 hours depending on type.
3. dosing systems for oxidizing agents
- Function: Precise dosing of hydrogen peroxide or ozone to maximize hydroxyl radical formation.
- Technology: Pumps or injectors for even distribution in the water flow.
4. control systems
- Sensor technology: Monitoring of UV intensity, water flow and dosing of chemicals.
- Automation: Control of operating parameters for maximum efficiency and safety.
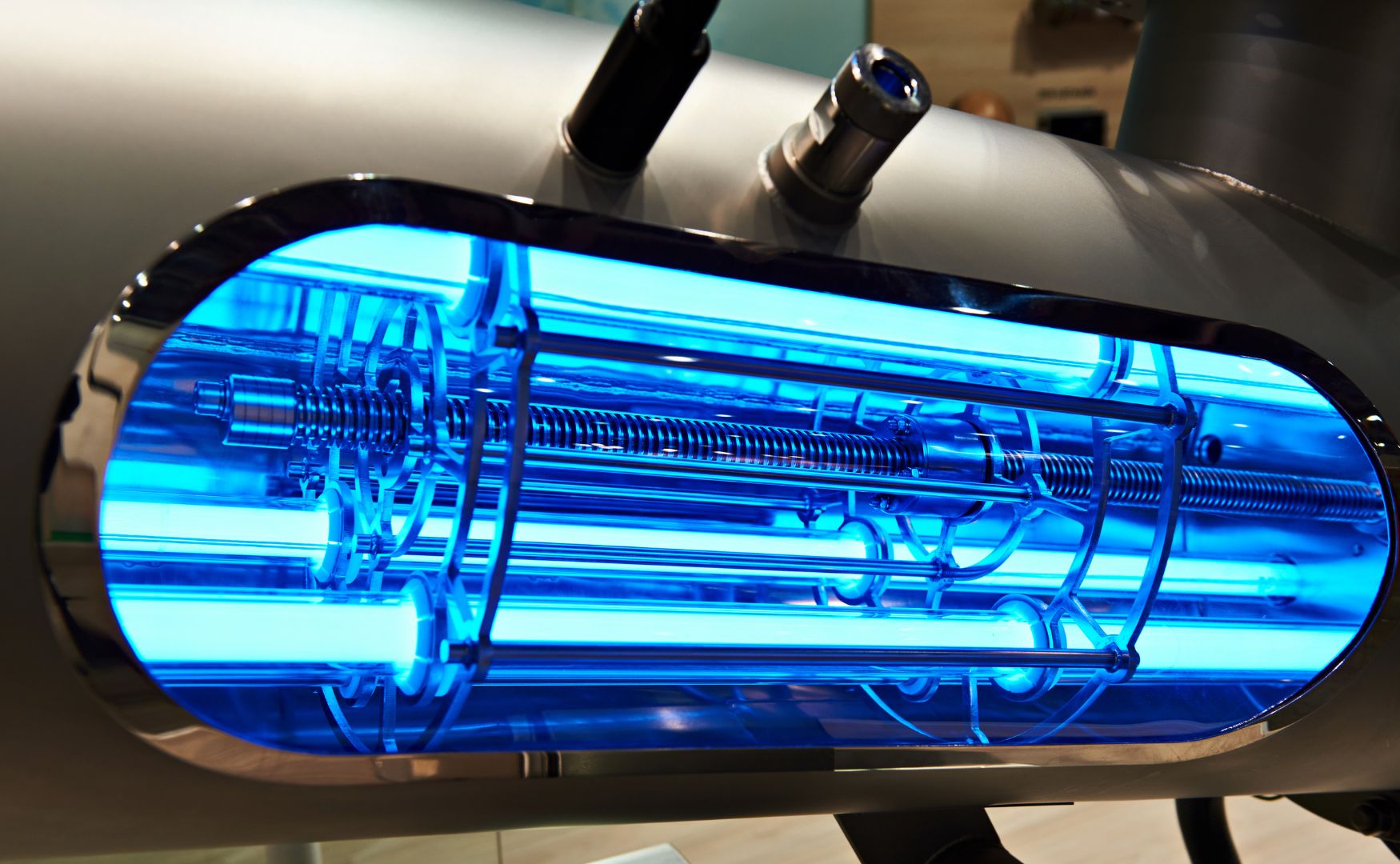
Photo: UV reactor with defined UV spectrum for the formation of highly radical hydroxyl radicals of the ALMA OXI UV
Applications of UV oxidation
UV oxidation is used in numerous industrial and municipal applications:
1. removal of micropollutants
- Examples:
- Degradation of hormones, pharmaceutical residues, pesticides and other organic micropollutants.
- Mechanism:
- Destruction of the molecular structures by hydroxyl radicals, so that the compounds are broken down into biodegradable fragments.
2. disinfection and inactivation of microorganisms
- Effect:
- Destruction of the DNA and RNA of microorganisms by UV radiation.
- Suitable for the inactivation of viruses, bacteria and protozoa.
3. color reduction and odor elimination
- Application:
- Textile industry for the removal of dyes.
- Food processing to neutralize odour-forming substances.
4. treatment of wastewater with a high COD value
- Mechanism:
- Oxidation of carbon compounds that increase the chemical oxygen demand (COD) to carbon dioxide and water.
5. treatment of process water
- Examples:
- Production of ultra-pure water for the semiconductor and pharmaceutical industries.
- Reduction of organic impurities that can cause fouling in membrane systems.
Advantages of UV oxidation
High efficiency:
- Fast reactions and high oxidizing power thanks to hydroxyl radicals.
Environmental friendliness:
- No use of chlorine, no formation of chlorinated by-products.
Broad field of application:
- Degradation of a variety of organic pollutants.
Compact system design:
- Suitable for limited space.
The challenges of UV oxidation
1. energy demand
- UV lamps have a high power consumption, especially in medium-pressure systems.
- Solution: Improving energy efficiency through optimized lamp designs and control systems.
2. turbidity and solids in the water
- Cloudy water reduces UV penetration and thus the efficiency of oxidation.
- Solution: Pre-filtration to remove solids.
3. formation of by-products
- In water containing bromide, UV oxidation can form bromates (BrO₃-), which are harmful to health.
- Solution: Optimization of the operating parameters and minimization of the bromide concentration.
Conclusion
UV oxidation is a highly effective technology for water and wastewater treatment that is characterized by its versatility, efficiency and environmental friendliness. It offers a sustainable solution for the removal of pollutants and microorganisms and is particularly suitable for applications where conventional processes reach their limits. With ongoing innovations in UV technology and the integration of energy-efficient components, UV oxidation will continue to play a central role in industrial water treatment in the future.
For further information on our products, please feel free to contact us at any time!







