Nitrification is a biological process in which ammonium (NH₄⁺) is converted into nitrite (NO₂-) and then into nitrate (NO₃-). This process is a central part of the nitrogen cycle and plays an important role in industrial wastewater treatment to break down nitrogen compounds and prevent them from being discharged into water bodies. Nitrogen compounds, especially ammonium, can have a toxic effect in high concentrations and pollute the environment, particularly through eutrophication (over-fertilization of water bodies), which can lead to oxygen deficiency and fish mortality.
Table of contents
Technical background and mode of operation
Nitrification takes place in two stages, which are carried out by specialized microorganisms:
1. ammonium oxidation:
- The first step is the oxidation of ammonium to nitrite. This process is catalyzed by ammonium-oxidizing bacteria (AOB) such as Nitrosomonas.
- Reaction equation:
- This step produces protons (H⁺), which lower the pH value of the water, which is why careful pH control is important.
2. nitrite oxidation:
- The second step is the oxidation of nitrite to nitrate, which is carried out by nitrite-oxidizing bacteria (NOB ) such as Nitrobacter.
- Reaction equation:
- Nitrate is less toxic than ammonium and nitrite and can be removed from wastewater by further processes such as denitrification.
Areas of application in wastewater treatment
Nitrification is an essential step in industrial and municipal wastewater treatment, especially in plants that have to process high nitrogen loads. Typical fields of application include
- Municipal wastewater treatment plants: Here, nitrification is often used in combination with denitrification to remove both ammonium and nitrate from the wastewater.
- Industrial wastewater: Nitrification is used in industries such as the food and beverage industry, the pharmaceutical industry and the chemical industry to reduce the nitrogen content in wastewater.
Operating conditions for nitrification
The following conditions must be met for effective nitrification:
- Oxygen supply: Nitrification is an aerobic process that requires sufficient oxygen. The oxygen content in the water must generally be above 2 mg/l.
- pH value: The pH value should be in the range of 6.5 to 8.5 to maximize the activity of the nitrifying bacteria. A pH value that is too low can inhibit the growth of the bacteria.
- Temperature: The optimum temperature for the nitrification process is between 15 °C and 30 °C. Below 10 °C, the process slows down considerably, while higher temperatures can promote bacterial growth.
- Carbon sources: Nitrification leads to a reduction in the alkalinity of the water, so it may be necessary to add alkalinity buffers or external carbon sources to keep the pH stable.

Photo: Denitrification and nitrification basin for industrial wastewater from the food industry in Chile, ALMA BHU BIO plant
The challenges of nitrification
One challenge in nitrification is the acidification of the water caused by the release of protons during ammonium oxidation. In industrial applications, it may therefore be necessary to use pH regulating agents to maintain the process efficiently. In addition, the process may run slower at low temperatures, which may require an additional heat source or special adjustments.
Advantages of nitrification
- Reduction of toxic nitrogen compounds: Ammonium and nitrite are toxic in high concentrations and can cause serious environmental damage. Nitrification helps to safely convert these compounds into nitrate, which can then be further treated or removed.
- Improved water quality: By removing ammonium and nitrite, nitrification significantly improves water quality, which is crucial for compliance with legal requirements for wastewater discharge.
- Adaptability: Nitrification can be adapted to a wide range of wastewater compositions and operating conditions and is a flexible process for various industrial applications.
ALMAWATECH solutions for nitrification
ALMAWATECH offers specially developed systems to optimize nitrification processes in industrial wastewater treatment plants. Our innovative solutions include:
ALMA BHU Bio
Our biologically activated activated sludge plant combines nitrification and denitrification in a modular system designed for large wastewater flows. This plant can process up to 500 m³/h of wastewater with a high nitrogen load. Nitrification takes place in the aerated stage, which is regulated by automatic control systems to optimize oxygenation and other parameters.
ALMA BioFil Compact
This compact biofiltration system for smaller wastewater flows (up to 100 m³/h) combines nitrification and denitrification in one efficient system. It is ideal as pre-treatment for downstream membrane systems and reduces nitrogen compounds that could lead to fouling in reverse osmosis systems.
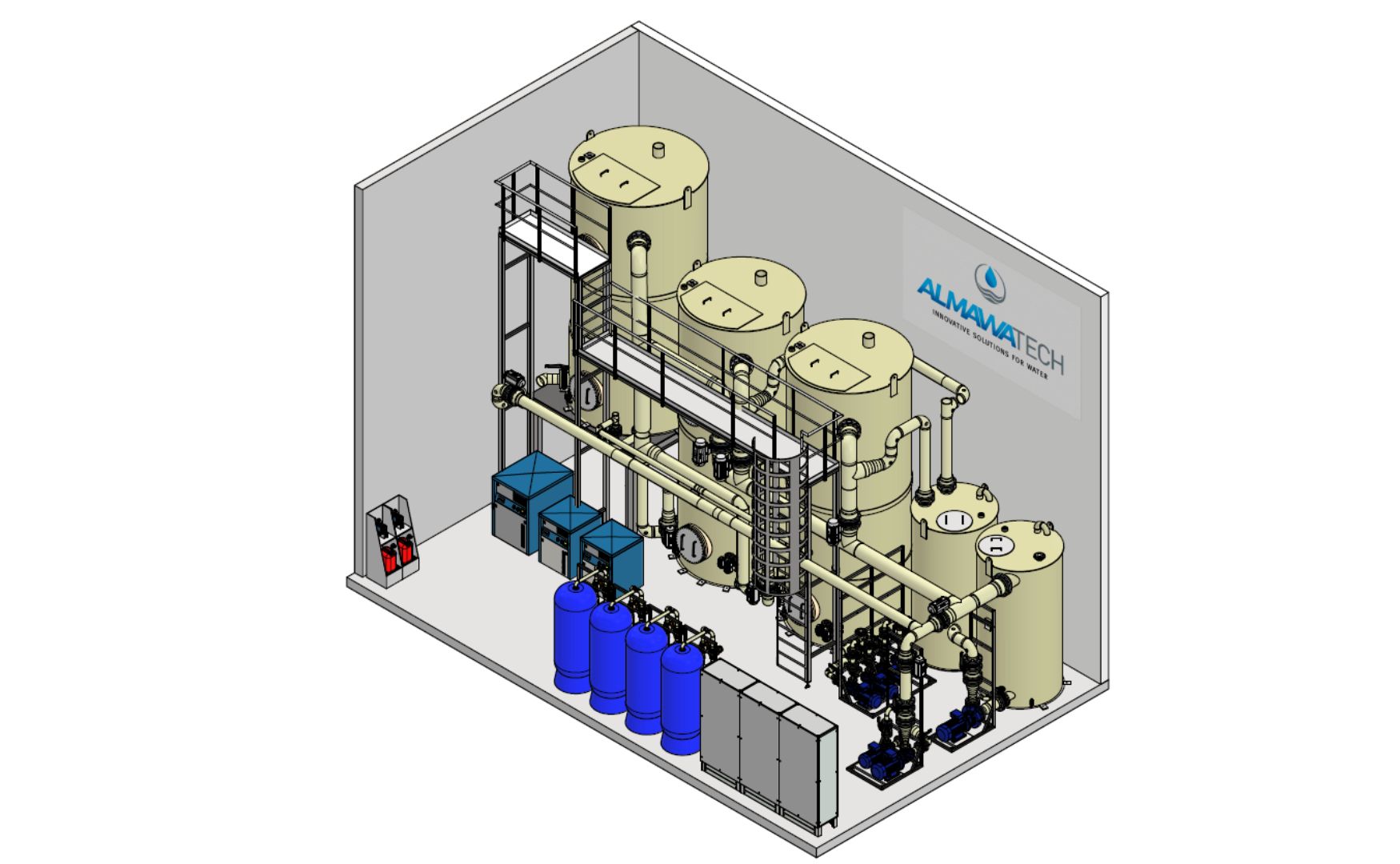
Photo: 3D design of our biologically activated ALMA BioFil Compact filtration system
ALMA BHU BAF
The large-scale concrete biofiltration plant offers efficient nitrification for wastewater flows of up to 1,000 m³/h. This plant uses an integrated system of aerobic and anoxic zones to maximize nitrogen removal. Particularly suitable for the food and beverage industry as well as municipal wastewater streams.

Photo: Photo of our biologically activated filtration, a combination process of mechanical cleaning and biodegradation(ALMA BHU BAF)
Conclusion
Nitrification is an indispensable biological process for removing nitrogen from wastewater, which protects the environment from harmful nitrogen compounds. With ALMAWATECH 's advanced technologies, nitrification processes can be optimally integrated into industrial wastewater treatment plants to achieve high efficiency and cost savings. Our customized plant solutions offer flexibility and reliability to meet the most demanding wastewater treatment requirements.








