Nitrate is a chemical compound and an end product in the biological nitrogen cycle that is produced by the oxidation process of ammonium and nitrite in wastewater. Nitrate plays a central role in industrial wastewater treatment, especially in nitrogen removal, in order to meet the legal requirements for wastewater discharge into water bodies. Although nitrate is less toxic than ammonium and nitrite, it is an environmental pollutant in high concentrations, particularly by promoting eutrophication (nutrient overload) in water bodies, which can increase algae growth and oxygen depletion.
Table of contents
Technical background and development
Nitrate is formed during nitrification, a biological process in which nitrogenous compounds are converted by microorganisms:
- Ammonium oxidation (first stage of nitrification):
- In the first phase of nitrification, ammonium (NH₄⁺) is oxidized to nitrite (NO₂-) by ammonium-oxidizing bacteria (AOB).
- Nitrite oxidation (second stage of nitrification):
- In the second stage, nitrite is oxidized to nitrate (NO₃-) by nitrite-oxidizing bacteria (NOB).
- Reaction equation:
Nitrate is considered to be relatively stable and accumulates in natural and artificial waters if it is not removed. It can accumulate heavily in drinking water and agricultural areas due to fertilizer inputs and inadequate treatment of wastewater.
Importance of nitrate in water treatment
In wastewater treatment, the removal of nitrate is becoming increasingly important as high nitrate levels can lead to pollution of water resources. Nitrate itself is harmful to health in high concentrations, especially in drinking water supplies, as it can lead to methemoglobinemia (also known as "blue baby syndrome") in infants. In the European Union, the limit value for nitrate in drinking water is 50 mg/l, which underlines the need for efficient nitrate removal from wastewater.
Nitrate removal in wastewater treatment
The removal of nitrate from wastewater is usually achieved by denitrification, a biological process in which nitrate is reduced to nitrogen gas (N₂) by denitrifying bacteria under anoxic conditions. This process is particularly efficient in anoxic reactors or wastewater treatment plants where carbon sources are added to provide the bacteria with the necessary substrate to reduce nitrate.

Photo: Denitrification and nitrification basin for industrial wastewater from the food industry in Chile, ALMA BHU BIO plant
Process of denitrification
Denitrification is a multi-stage biological process in which nitrate is reduced to nitrogen gas (N₂) by various intermediate products (NO₂-, NO, N₂O). Denitrification takes place according to the following reaction chain:
This process requires anoxic conditions, i.e. a lack of oxygen, as well as an organic carbon source (e.g. methanol, ethanol or acetate) to feed the denitrifying microorganisms.
Challenges and operating conditions for nitrate removal
Lack of oxygen: Denitrification can only take place in an environment where there is no free oxygen. A lack of oxygen ensures that the microorganisms use nitrate as an electron acceptor.
Carbon source: As the denitrification process requires an organic carbon source, the addition of substrates is often necessary if the wastewater does not contain sufficient organic carbon.
Temperature: Denitrification is highly temperature-dependent. The optimum temperature is between 15 °C and 30 °C, although colder conditions can slow down the process considerably.
Environmental relevance of nitrate
- Eutrophication: Nitrate contributes significantly to the over-fertilization of water bodies, which promotes the growth of algae and other aquatic plants. This leads to a lack of oxygen in the affected water bodies and endangers aquatic life.
- Drinking water pollution: In agricultural regions, nitrate from fertilizers can enter the groundwater, which endangers the drinking water supply. Effective wastewater treatment processes are required here to remove the nitrate before it is discharged.
ALMAWATECH solutions for nitrate removal
ALMAWATECH offers customized solutions for the efficient removal of nitrate from industrial wastewater. These include
ALMA BHU Bio
Our biologically activated activated sludge plant combines nitrification and denitrification in a modular system designed for large wastewater flows. This plant can process up to 500 m³/h of wastewater with a high nitrogen load. Nitrification takes place in the aerated stage, which is regulated by automatic control systems to optimize oxygenation and other parameters.
ALMA BioFil Compact
This compact biofiltration system for smaller wastewater flows (up to 100 m³/h) combines nitrification and denitrification in one efficient system. It is ideal as pre-treatment for downstream membrane systems and reduces nitrogen compounds that could lead to fouling in reverse osmosis systems.
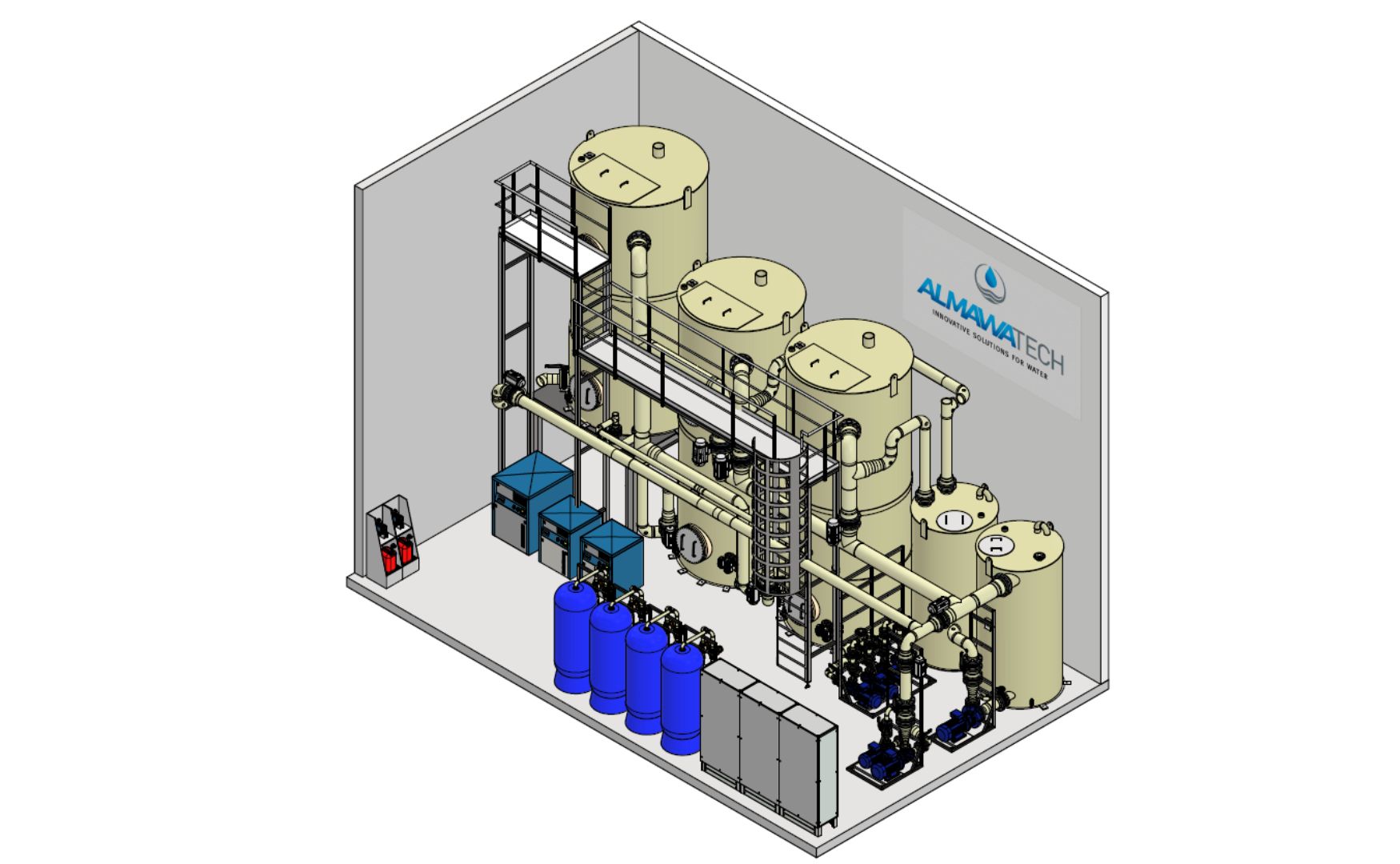
Photo: 3D design of our biologically activated ALMA BioFil Compact filtration system
ALMA BHU BAF
The large-scale concrete biofiltration plant offers efficient nitrification for wastewater flows of up to 1,000 m³/h. This plant uses an integrated system of aerobic and anoxic zones to maximize nitrogen removal. Particularly suitable for the food and beverage industry as well as municipal wastewater streams.

Photo: Photo of our biologically activated filtration, a combination process of mechanical cleaning and biodegradation(ALMA BHU BAF)
Conclusion
Nitrate is an end product in the biological nitrogen cycle that can be harmful to the environment and human health in high concentrations. The removal of nitrate from wastewater is crucial to protect the environment and comply with legal limits. With ALMAWATECH 's advanced technologies, nitrification and denitrification processes can be efficiently integrated into industrial wastewater treatment plants to ensure complete nitrogen removal.