Alkalinity describes the ability of water to neutralize acids and is determined by the concentration of hydrogen carbonate (HCO₃-), carbonate (CO₃²-) and, to a lesser extent, hydroxide ions (OH-). It is a measure of the buffering capacity of water and indicates how well it is able to keep the pH value stable when acids are added. In industrial water treatment and wastewater treatment, alkalinity is an important parameter for controlling the addition of chemicals, ensuring corrosion protection and stabilizing biological processes in wastewater treatment.
Table of contents
Technical background
Alkalinity is expressed in millival per liter (mval/L) or in mg/L CaCO₃ (calcium carbonate). It is made up of various alkaline compounds that are able to bind hydrogen ions (H⁺) and thus buffer the pH value of the water. The main components of alkalinity in water are
- Hydrogen carbonate (HCO₃-): The most common contributor to alkalinity in natural water sources, especially groundwater and surface waters.
- Carbonate (CO₃²-): Occurs mainly in water with a higher pH value (> 8.3) and contributes significantly to alkalinity.
- Hydroxide (OH-): Occurs mainly in strongly alkaline water samples and has an additional buffering effect at high pH values.
Alkalinity is not the same as pH, although it is related to it. While the pH value indicates the acidity of the water, the alkalinity describes the ability of the water to buffer the pH value when acids are added.
Importance of alkalinity in industrial water and wastewater treatment
Alkalinity is of great importance in industrial water treatment and wastewater treatment, as it influences the stability of chemical processes, corrosion protection in plants and the efficiency of biological processes. Stable alkalinity ensures that the pH value of the water or wastewater does not fluctuate greatly, even when chemicals are added or during biological degradation processes. This is particularly important in various areas of application:
1. biological wastewater treatment
In biological activated sludge plants, alkalinity plays an important role in the nitrification process, in which ammonium (NH₄⁺) is converted into nitrate (NO₃-). This process releases hydrogen ions, which can lower the pH value. Sufficient alkalinity ensures that the pH value remains in the optimum range and that the microorganisms can continue to work effectively. Without sufficient buffering capacity, the pH value can drop too low, which inhibits biological activity and impairs the degradation of pollutants.
2. corrosion protection
In cooling water circuits and hot water systems, alkalinity is a decisive factor for corrosion protection. Too low an alkalinity value leads to an uncontrolled drop in pH, which favors corrosive conditions. Alkalinity values that are too high, on the other hand, can lead to scale formation. Therefore, the alkalinity in such circuits must be closely monitored and regulated by adding chemicals such as lime (CaCO₃) or sodium carbonate (Na₂CO₃) to ensure optimum water quality and equipment protection.
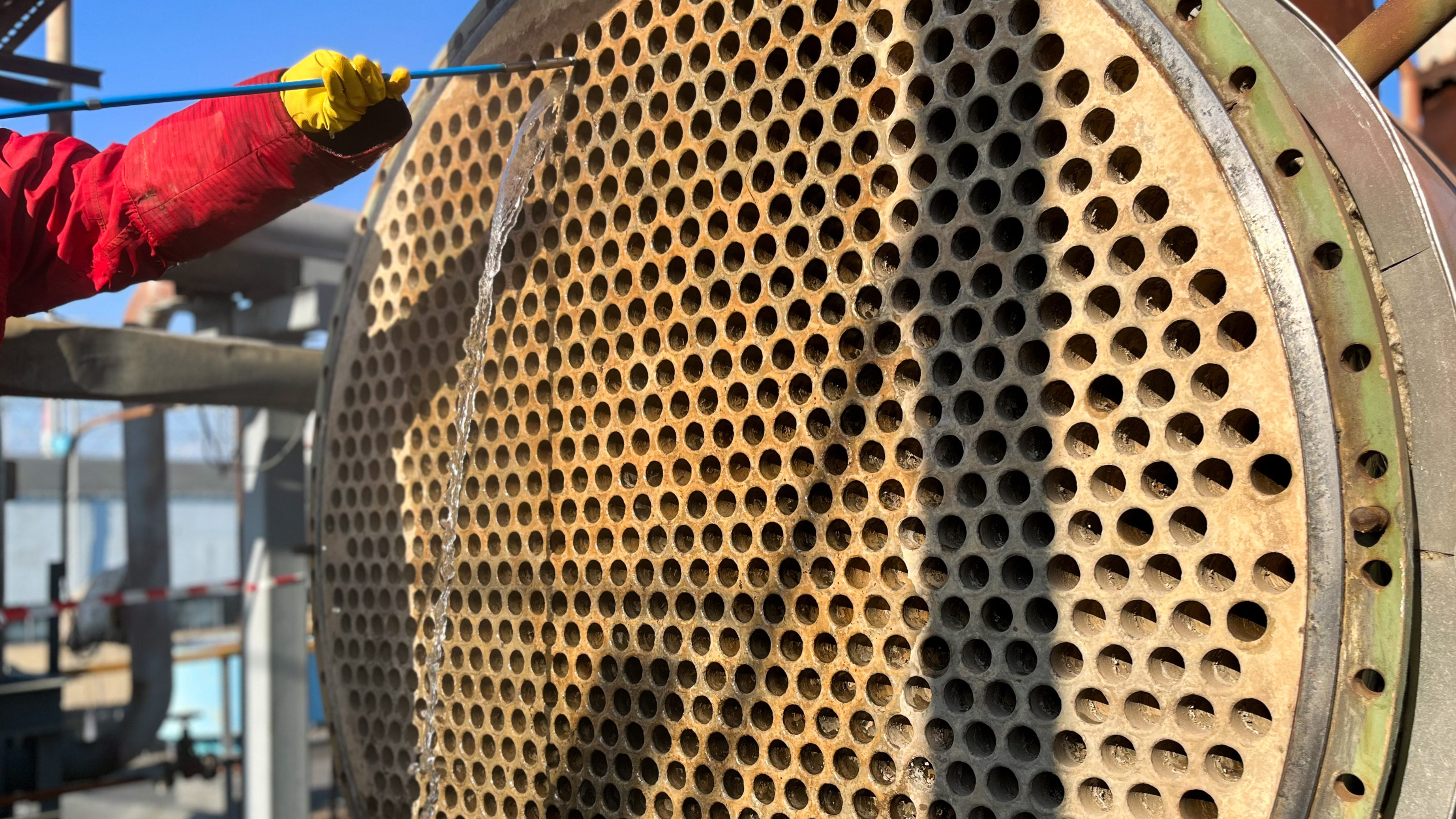
Photo: Corrosion in steam boiler systems can cause considerable damage and impair operating efficiency. For more information on our corrosion inhibitors, alkalizing agents and oxygen binding agents, visit our website and discover our operating fluids for boiler systems(ALMA AQUA boiler systems) and cooling water circuits(ALMA AQUA cooling water).
3. precipitation and neutralization processes
In many industrial wastewater treatment processes, chemicals are used to regulate the pH value and precipitate pollutants. Alkalinity determines how much acid or base needs to be added to bring the pH into the desired range. A high alkalinity reduces the amount of acid required for neutralization and allows for more efficient pH control.

Photo: Automatic dosing station for acid and lye for pH-value-controlled neutralization of wastewater(ALMA Neutra)
Influence of alkalinity on the design of water treatment plants
The design of water treatment and wastewater treatment plants must take into account the alkalinity of the water to be treated. Especially when planning neutralization plants, precipitation reactors and biological treatment processes, a precise determination of the alkalinity is necessary to ensure that the system functions properly.
- Neutralization systems: A high alkalinity can reduce the amount of acid required for neutralization, which lowers operating costs.
- Precipitation processes: The buffer capacity of the water influences the quantity of precipitants and the efficiency of the precipitation.
- Biological plants: Sufficient alkalinity is necessary to protect the microorganisms in activated sludge reactors or biofilters from pH value fluctuations.
Measurement and monitoring of alkalinity
Alkalinity is usually determined by titration, in which an acid is added to a water sample until a certain pH value is reached. The amount of acid consumed gives an indication of the buffering capacity of the water. Continuous monitoring of alkalinity is important in industrial applications to ensure that processes are stable and efficient.
Conclusion
Alkalinity is an important parameter in industrial water and wastewater treatment, which describes the buffering capacity of the water and ensures that the pH value remains stable. Stable alkalinity is particularly important for biological degradation processes, corrosion protection and chemical precipitation and neutralization processes. By properly monitoring and regulating alkalinity, the efficiency of water treatment plants can be maximized and operation optimized.